Properties of Solutions Chapter 11 Solutions Solutions Solutions






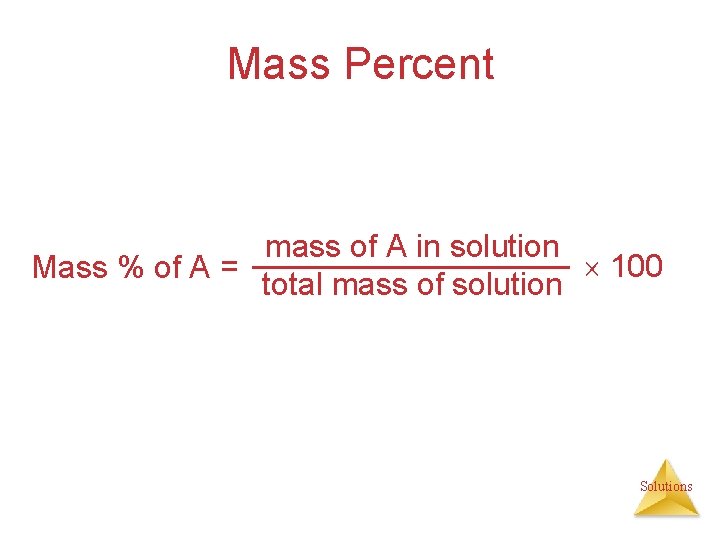












































- Slides: 99

Properties of Solutions Chapter 11 Solutions

Solutions • Solutions are homogeneous mixtures of two or more pure substances in a SINGLE phase. • In a solution, the solute is dispersed uniformly throughout the solvent. Solutions

TERMS TO KNOW • Solubility — maximum amount of material that will dissolve in a given amount of solvent at a given temperature to produce a stable solution. In other words, the solution is saturated. Study the solubility rules!! • Miscible — When two or more liquids mix (ex. Water and food coloring) • Immiscible — When two or more liquids DON’T mix. —they usually layer if allowed to set for a while. (ex. water and oil) Solutions

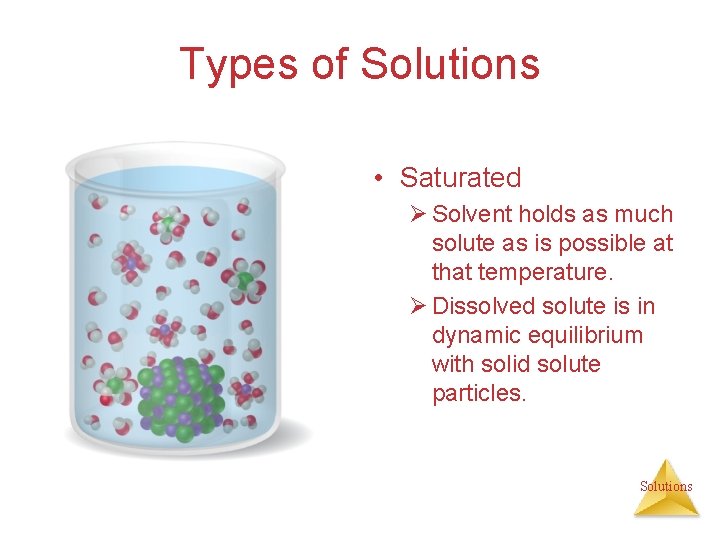
Types of Solutions • Saturated Ø Solvent holds as much solute as is possible at that temperature. Ø Dissolved solute is in dynamic equilibrium with solid solute particles. Solutions

Types of Solutions • Unsaturated Ø Less than the maximum amount of solute for that temperature is dissolved in the solvent. Solutions

Types of Solutions • Supersaturated Ø Solvent holds more solute than is normally possible at that temperature. Ø These solutions are unstable; crystallization can usually be stimulated by adding a “seed crystal” or scratching the side of the flask. Solutions

Ways of Expressing Concentrations of Solutions

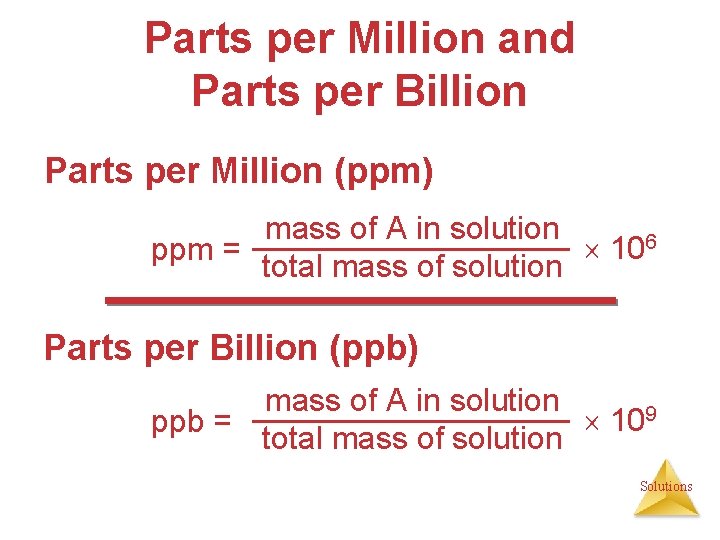
Parts per Million and Parts per Billion Parts per Million (ppm) mass of A in solution 106 ppm = total mass of solution Parts per Billion (ppb) mass of A in solution 109 ppb = total mass of solution Solutions

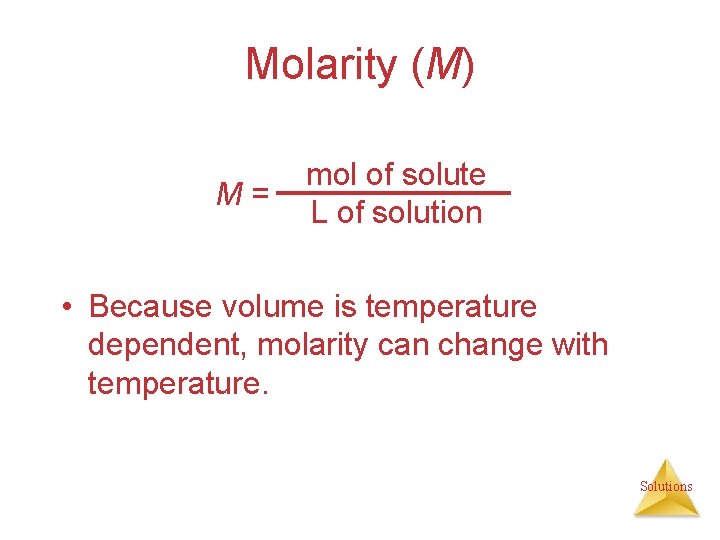
Molarity (M) M= mol of solute L of solution • Because volume is temperature dependent, molarity can change with temperature. Solutions
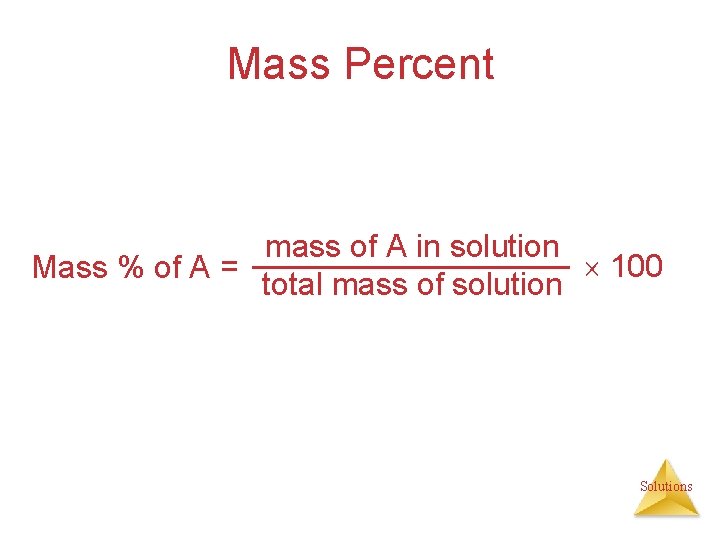
Mass Percent mass of A in solution 100 Mass % of A = total mass of solution Solutions

Mole Fraction (X) moles of A XA = total moles in solution • In some applications, one needs the mole fraction of solvent, not solute— make sure you find the quantity you need! Solutions

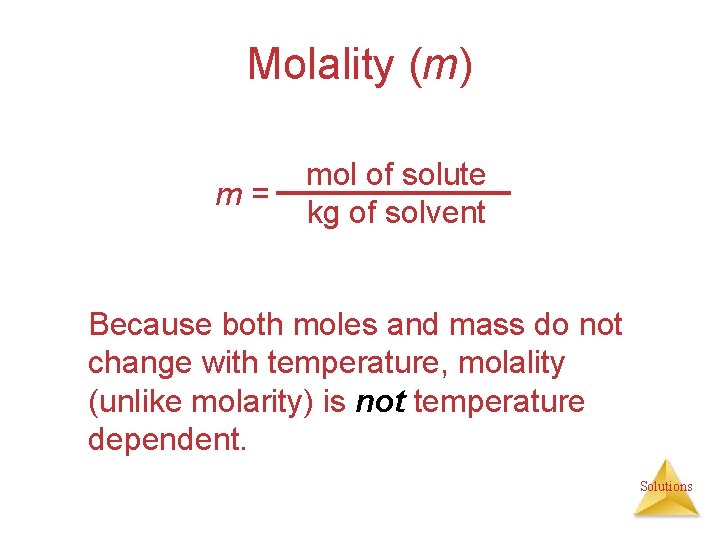
Molality (m) m= mol of solute kg of solvent Because both moles and mass do not change with temperature, molality (unlike molarity) is not temperature dependent. Solutions

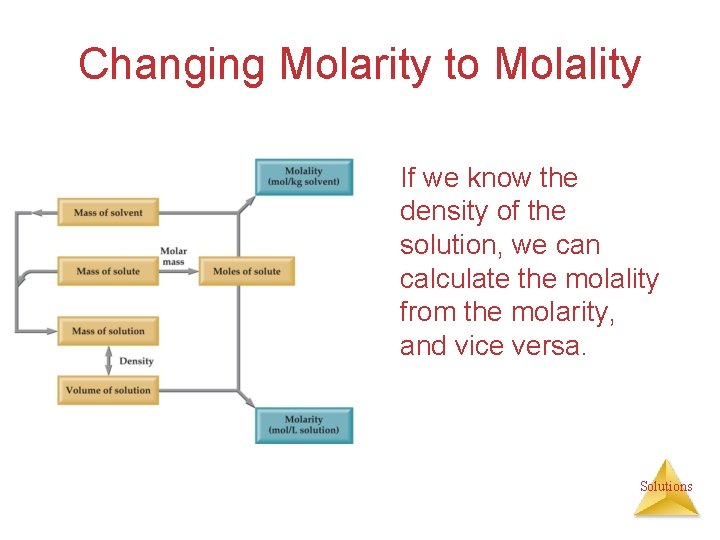
Changing Molarity to Molality If we know the density of the solution, we can calculate the molality from the molarity, and vice versa. Solutions

PRACTICE ONE A solution is prepared by mixing 1. 00 g ethanol (C 2 H 5 OH) with 100. 0 g water to give a final volume of 101 m. L. Calculate the molarity, mass percent, mole fraction, and molality of ethanol in this solution. Solutions

PRACTICE TWO The electrolyte in automobile lead storage batteries is a 3. 75 M sulfuric acid solution that has a density of 1. 230 g/m. L. Calculate the mass percent and molality of the sulfuric acid. Solutions

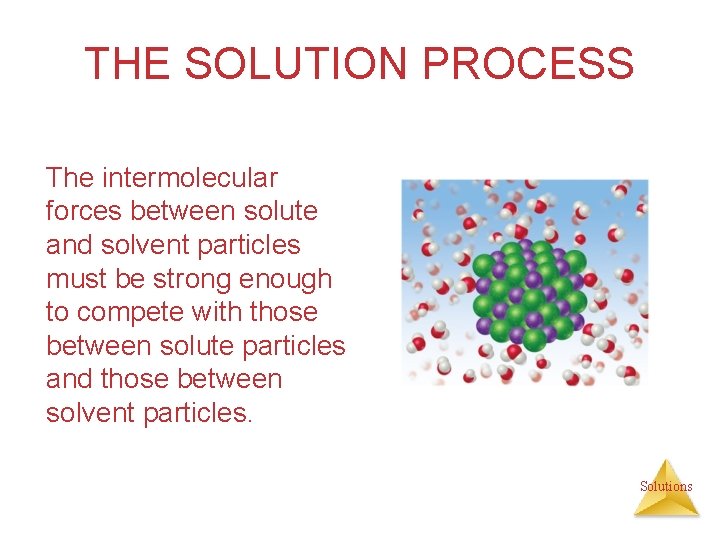
THE SOLUTION PROCESS The intermolecular forces between solute and solvent particles must be strong enough to compete with those between solute particles and those between solvent particles. Solutions

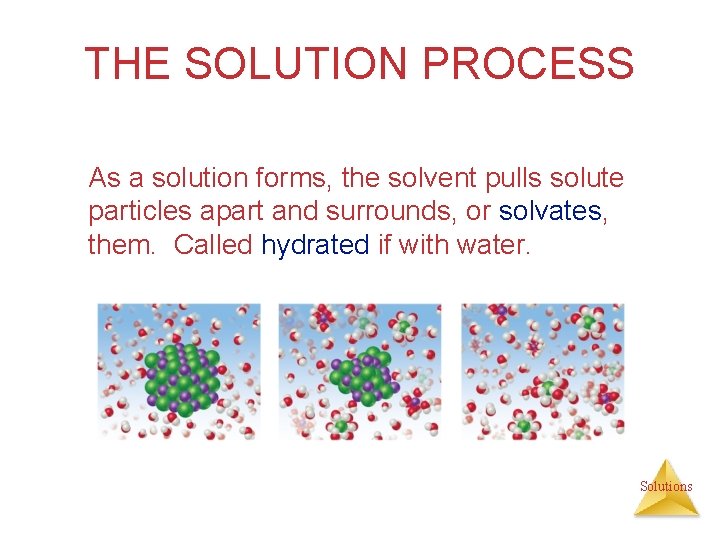
THE SOLUTION PROCESS As a solution forms, the solvent pulls solute particles apart and surrounds, or solvates, them. Called hydrated if with water. Solutions

THE SOLUTION PROCESS • REMEMBER: “Like dissolves like” – Polar solutes dissolve in polar solvents because the attraction to the solvent by the solute is greater than to itself. – Nonpolar solutes dissolve in nonpolar solvents because no one is attraction to anyone so they randomly disperse. Solutions

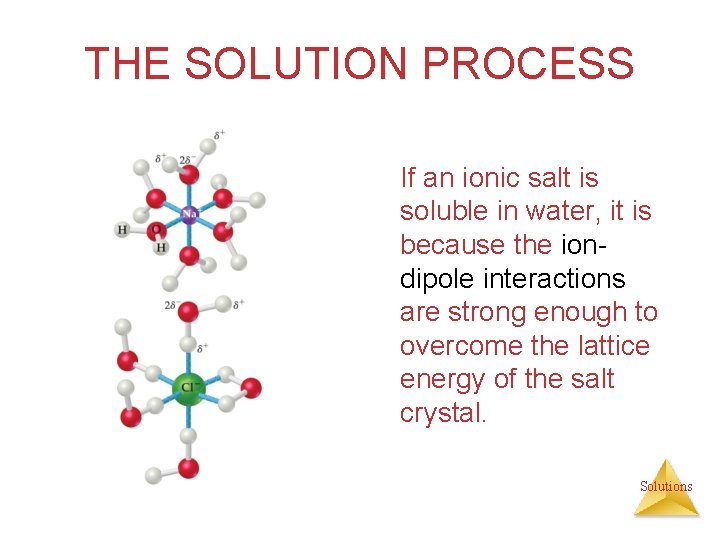
THE SOLUTION PROCESS If an ionic salt is soluble in water, it is because the iondipole interactions are strong enough to overcome the lattice energy of the salt crystal. Solutions

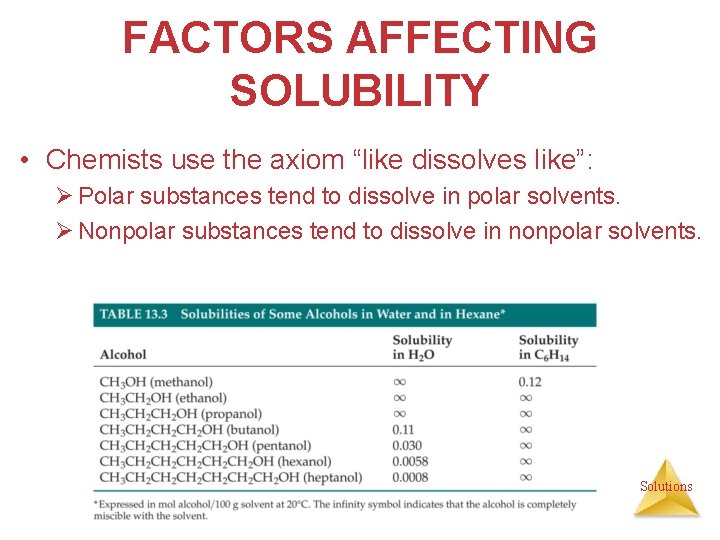
THE SOLUTION PROCESS • Solubilities of alcohols in water: As the hydrocarbon portion of the alcohol increases in length, the alcohol becomes less soluble. More of the molecule is nonpolar; the dipole moment is diminished. • Solubilities of alcohol in nonpolar solvents: As the hydrocarbon portion of the alcohol increases in length, the alcohol becomes more soluble in a nonpolar solvent such as hexane. Solutions

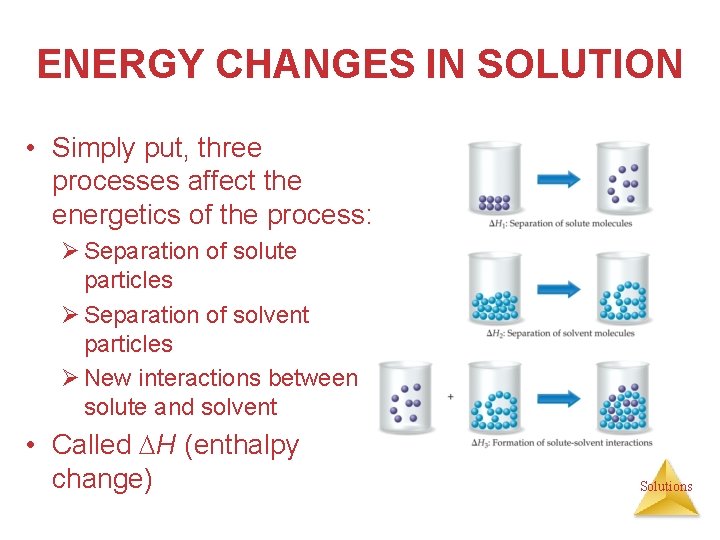
ENERGY CHANGES IN SOLUTION • Simply put, three processes affect the energetics of the process: Ø Separation of solute particles Ø Separation of solvent particles Ø New interactions between solute and solvent • Called H (enthalpy change) Solutions

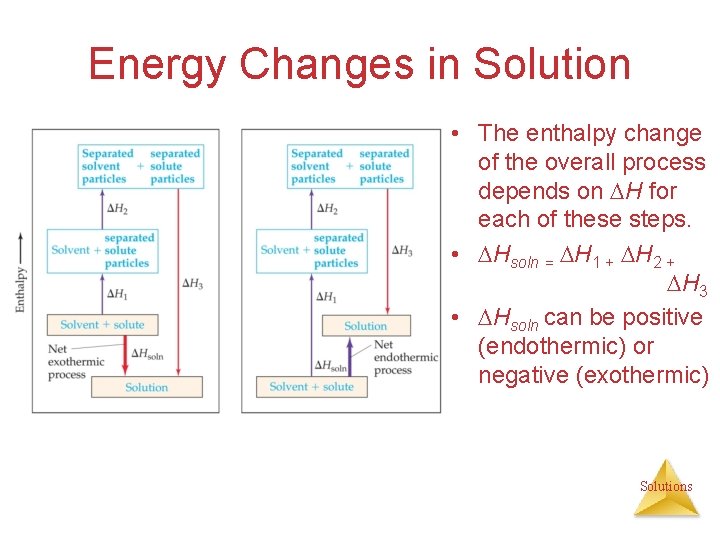
Energy Changes in Solution • The enthalpy change of the overall process depends on H for each of these steps. • Hsoln = H 1 + H 2 + H 3 • Hsoln can be positive (endothermic) or negative (exothermic) Solutions

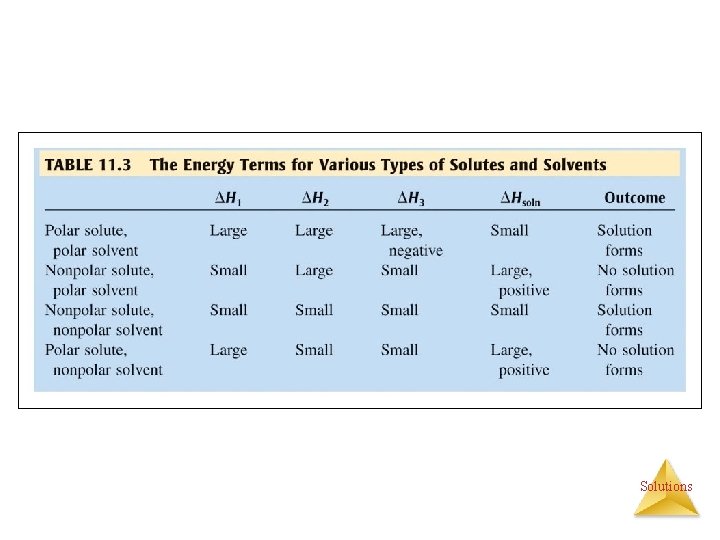
STEP ONE • Separating the solute into individual components of the solute (expanding the solute). This requires ENERGY be added to the system, therefore endothermic. The magnitude of the value is high in ionic and polar solutes, low in nonpolar solutes. • ΔHsolute = −ΔHlattice energy Solutions

STEP TWO • Overcoming IMFs in solvent to make room for the solute (expanding the solvent). Requires that ENERGY be added to the system, therefore endothermic. The magnitude of the value is high in polar solvents, low in nonpolar solvents. Solutions

STEP THREE • Interaction of solute and solvent to form the solution. • Energy must be released here, else the solution would never form since nature always tends toward a lower energy state, therefore exothermic. The magnitude of this value is high in polar solute — polar solvent interactions, low in other types of interactions. Solutions

ENTHALPY OF HYDRATION • ΔH 2 + ΔH 3 = enthalpy of hydration (ΔHhyd) • Enthalpy of hydration is more negative for small ions and highly charged ions. – Some heats of solution are + (endothermic). The solution process involves two factors; the change in heat and the change in entropy, and the relative magnitude of these two factors determine whether a solute dissolves in a solvent. Solutions

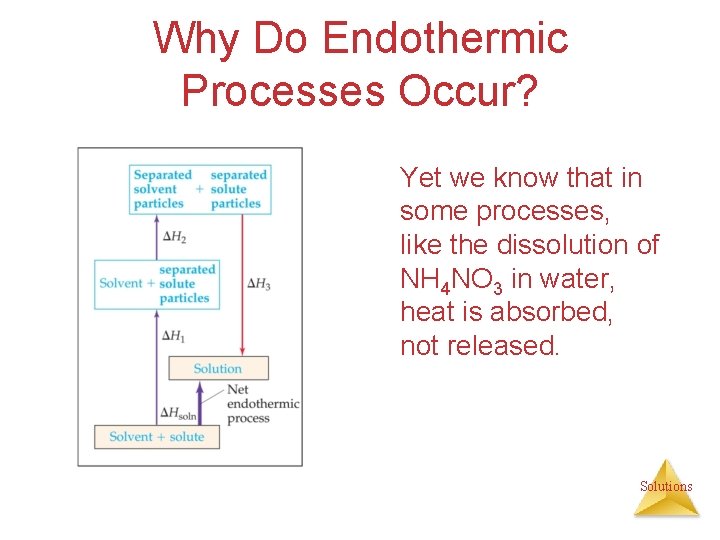
Why Do Endothermic Processes Occur? Things do not tend to occur spontaneously (i. e. , without outside intervention) unless the energy of the system is lowered. Solutions

Why Do Endothermic Processes Occur? Yet we know that in some processes, like the dissolution of NH 4 NO 3 in water, heat is absorbed, not released. Solutions

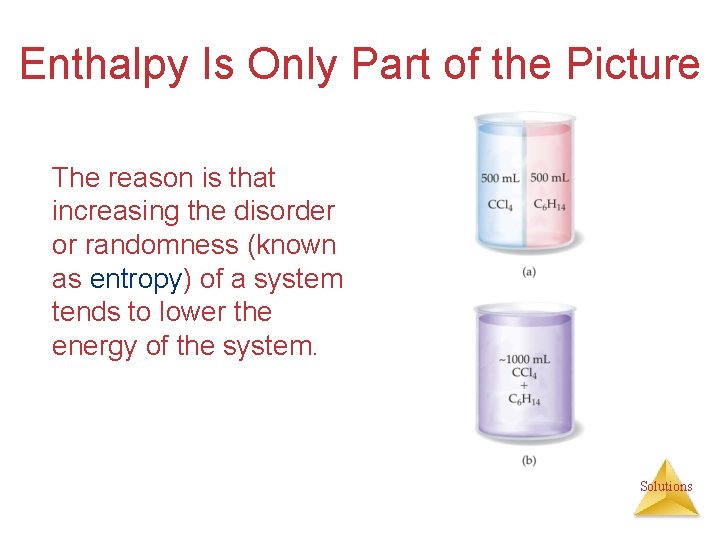
Enthalpy Is Only Part of the Picture The reason is that increasing the disorder or randomness (known as entropy) of a system tends to lower the energy of the system. Solutions

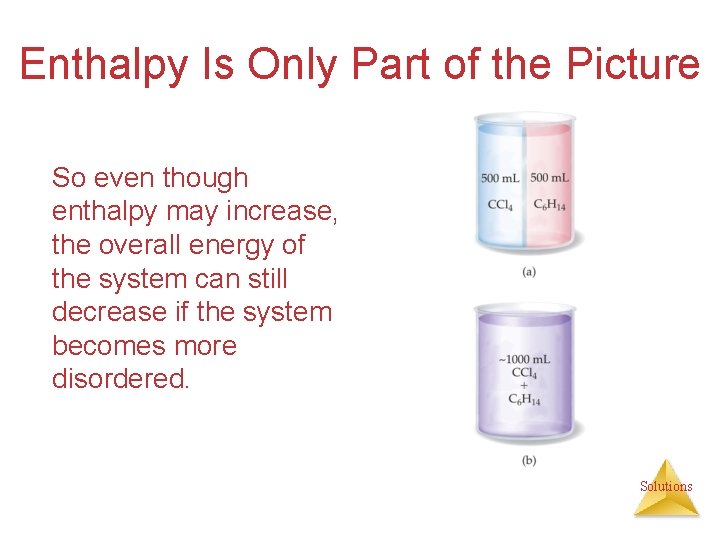
Enthalpy Is Only Part of the Picture So even though enthalpy may increase, the overall energy of the system can still decrease if the system becomes more disordered. Solutions

Solutions

PRACTICAL USE Hot and Cold packs • These often consist of a heavy outer pouch containing water and a thin inner pouch containing a salt. A squeeze on the outer pouch breaks the inner pouch and the salt dissolves. • Some hot packs use anhydrous Ca. Cl 2 (ΔHsoln = − 82. 8 k. J/mol) whereas many cold packs use NH 4 NO 3 (ΔHsoln = +25. 7 k. J/mol). Solutions

PRACTICE THREE • Decide whether liquid hexane (C 6 H 14) or liquid methanol (CH 3 OH) is the more appropriate solvent for the substances grease (C 20 H 42) and potassium iodide (KI). Solutions

STUDENT, BEWARE! Just because a substance disappears when it comes in contact with a solvent, it doesn’t mean the substance dissolved. Solutions

STUDENT, BEWARE! • Dissolution is a physical change—you can get back the original solute by evaporating the solvent. • If you can’t, the substance didn’t dissolve, it reacted. Solutions

FACTORS AFFECTING SOLUBILITY • Chemists use the axiom “like dissolves like”: Ø Polar substances tend to dissolve in polar solvents. Ø Nonpolar substances tend to dissolve in nonpolar solvents. Solutions

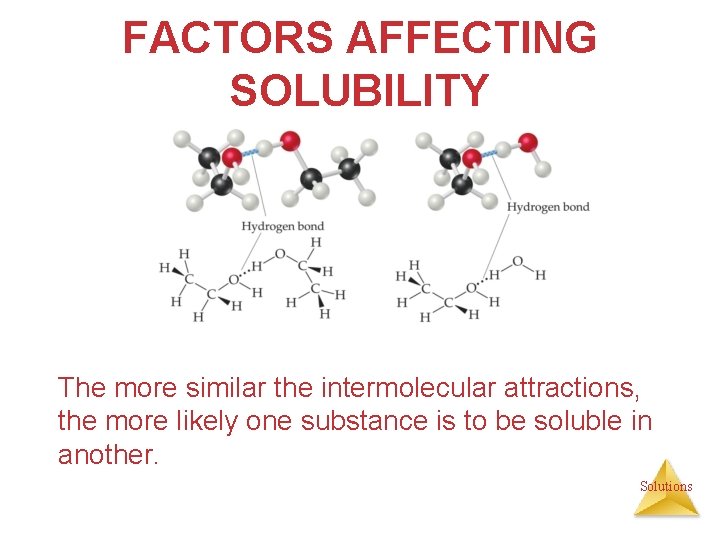
FACTORS AFFECTING SOLUBILITY The more similar the intermolecular attractions, the more likely one substance is to be soluble in another. Solutions

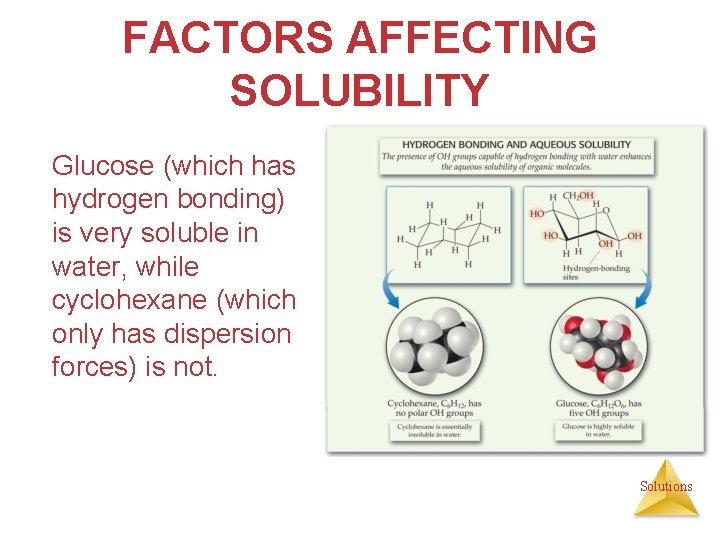
FACTORS AFFECTING SOLUBILITY Glucose (which has hydrogen bonding) is very soluble in water, while cyclohexane (which only has dispersion forces) is not. Solutions

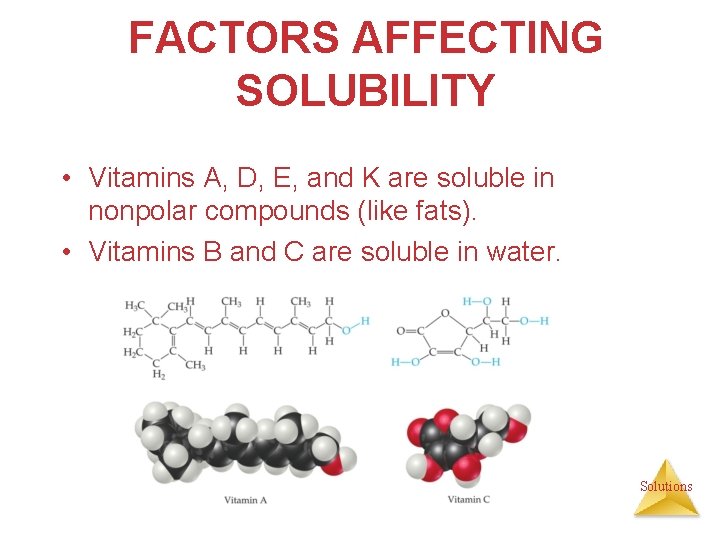
FACTORS AFFECTING SOLUBILITY • Vitamins A, D, E, and K are soluble in nonpolar compounds (like fats). • Vitamins B and C are soluble in water. Solutions

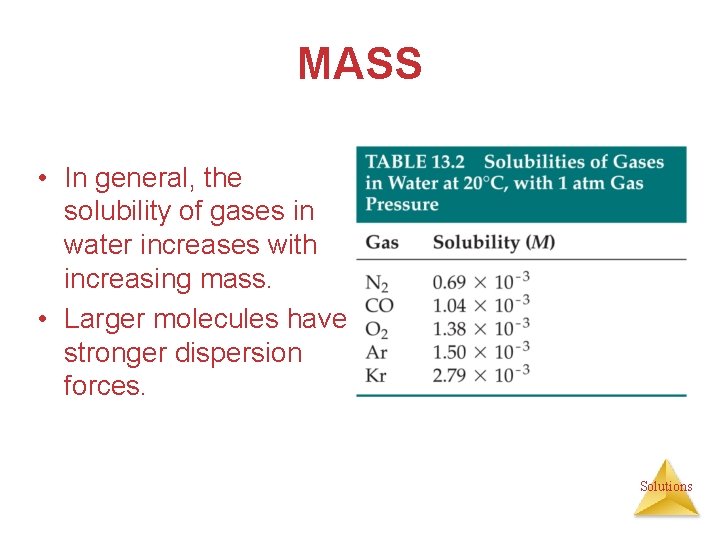
MASS • In general, the solubility of gases in water increases with increasing mass. • Larger molecules have stronger dispersion forces. Solutions

PRESSURE • The solubility of liquids and solids does not change appreciably with pressure. • The solubility of a gas in a liquid is directly proportional to its pressure. Solutions

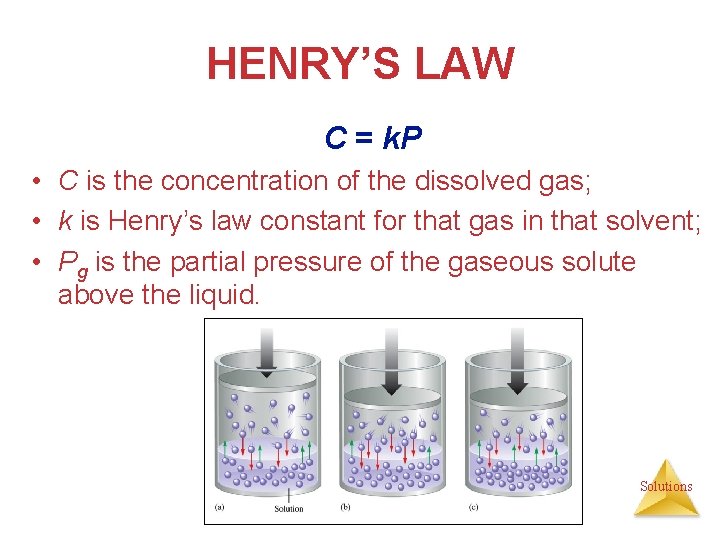
HENRY’S LAW • the amount of a gas dissolved in a solution is directly proportional to the pressure of the gas above the solution. • Henry’s Law is obeyed best for dilute solutions of gases that do not dissociate or react with the solvent. Solutions

HENRY’S LAW C = k. P • C is the concentration of the dissolved gas; • k is Henry’s law constant for that gas in that solvent; • Pg is the partial pressure of the gaseous solute above the liquid. Solutions

PRACTICE FOUR A certain soft drink is bottled so that a bottle at 25ºC contains CO 2 gas at a pressure of 5. 0 atm over the liquid. Assuming that the partial pressure of CO 2 in the atmosphere is 4. 0 × 10− 4 atm, calculate the equilibrium concentrations of CO 2 in the soda both before and after the bottle is opened. The Henry’s law constant for CO 2 in aqueous solution is 0. 031 mol/L • atm at 25ºC. Solutions

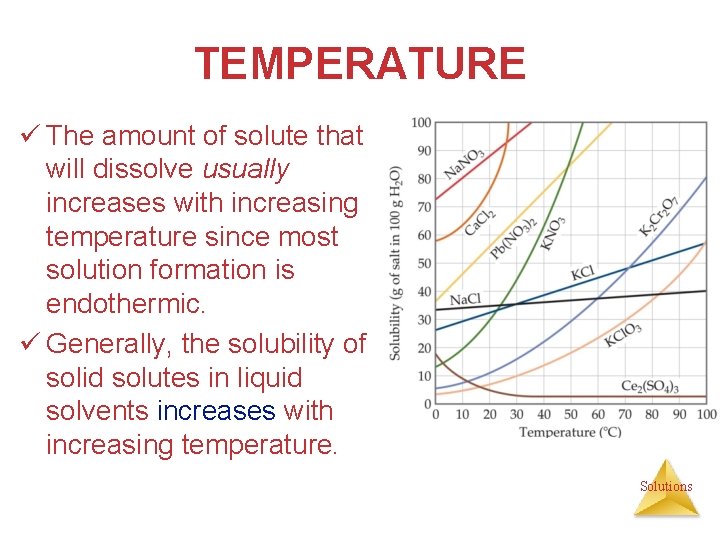
TEMPERATURE ü The amount of solute that will dissolve usually increases with increasing temperature since most solution formation is endothermic. ü Generally, the solubility of solid solutes in liquid solvents increases with increasing temperature. Solutions

TEMPERATURE • Solubility generally increases with temperature if the solution process is endothermic (ΔHsoln > 0). • Solubility generally decreases with temperature if the solution process is exothermic (ΔHsoln < 0). • This can be explained by Le. Chatelier’s Principle. Solutions

TEMPERATURE • The opposite is true of gases: Ø Carbonated soft drinks are more “bubbly” if stored in the refrigerator. Ø Warm lakes have less O 2 dissolved in them than cool lakes. Solutions

COLLIGATIVE PROPERTIES • Changes in colligative properties depend only on the number of solute particles present, not on the identity of the solute particles. • IMFs of solvent are interrupted by solute. • Among colligative properties are ØVapor pressure lowering ØBoiling point elevation ØMelting point depression ØOsmotic pressure Solutions

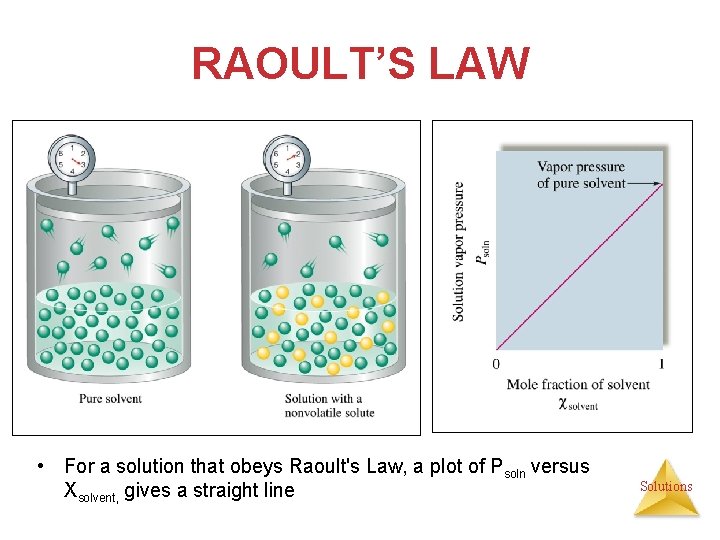
VAPOR PRESSURE Because of solutesolvent intermolecular attraction, higher concentrations of nonvolatile solutes make it harder for solvent to escape to the vapor phase. Solutions

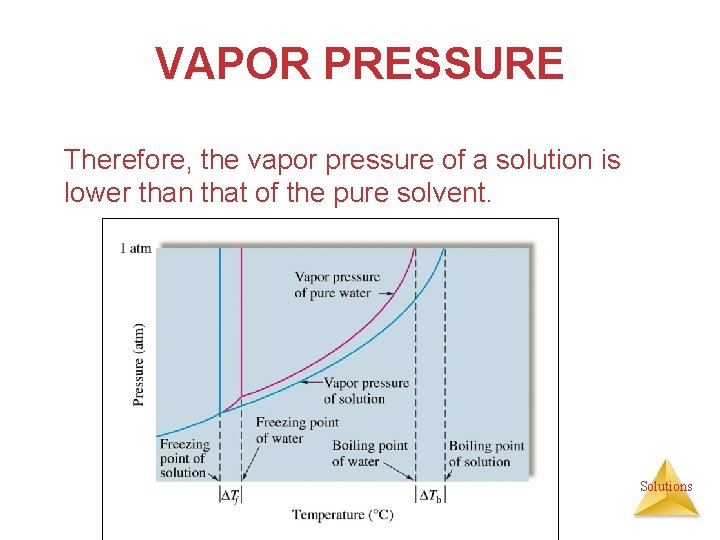
VAPOR PRESSURE Therefore, the vapor pressure of a solution is lower than that of the pure solvent. Solutions

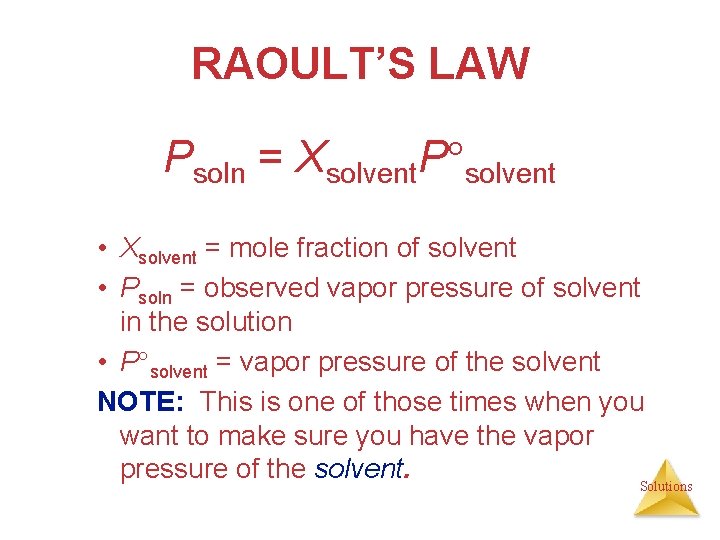
RAOULT’S LAW Psoln = Xsolvent. P solvent • Xsolvent = mole fraction of solvent • Psoln = observed vapor pressure of solvent in the solution • P solvent = vapor pressure of the solvent NOTE: This is one of those times when you want to make sure you have the vapor pressure of the solvent. Solutions

RAOULT’S LAW • The vapor pressure of a solution is directly proportional to the mole fraction of solvent present. • If the solute ionizes, the number of ions further lowers the vapor pressure. Solutions

RAOULT’S LAW • The moles of solute must be multiplied by the number of ions the given solute breaks into. • For instance, if we had one mole of Na. Cl as the solute, we would use two moles of particles for our mole fraction calculations; Al. Cl 3 would yield a van’t Hoff factor of four, etc. • For nonelectrolytes, i = 1. For electrolytes, i = the number of particles formed when one formula unit of the solute dissolves in the Solutions solvent.

RAOULT’S LAW • For a solution that obeys Raoult's Law, a plot of Psoln versus Xsolvent, gives a straight line Solutions

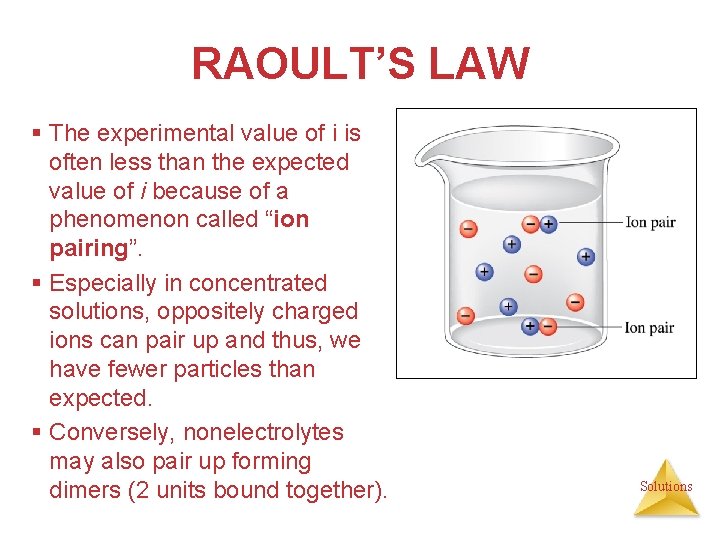
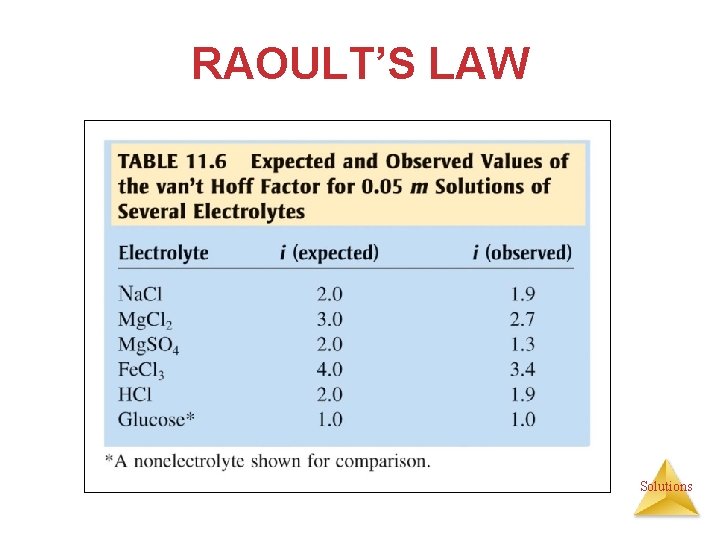
RAOULT’S LAW § The experimental value of i is often less than the expected value of i because of a phenomenon called “ion pairing”. § Especially in concentrated solutions, oppositely charged ions can pair up and thus, we have fewer particles than expected. § Conversely, nonelectrolytes may also pair up forming dimers (2 units bound together). Solutions

RAOULT’S LAW § An ideal solution is a solution that obeys Raoult’s Law. There is no such thing. § In very dilute solutions, Raoult’s Law works fairly well. Solutions are most ideal when the solute and the solvent are very similar. § If hydrogen bonding occurs between solute and solvent, vapor pressure is less than expected. We call this a negative deviation from Raoult’s law. Solutions

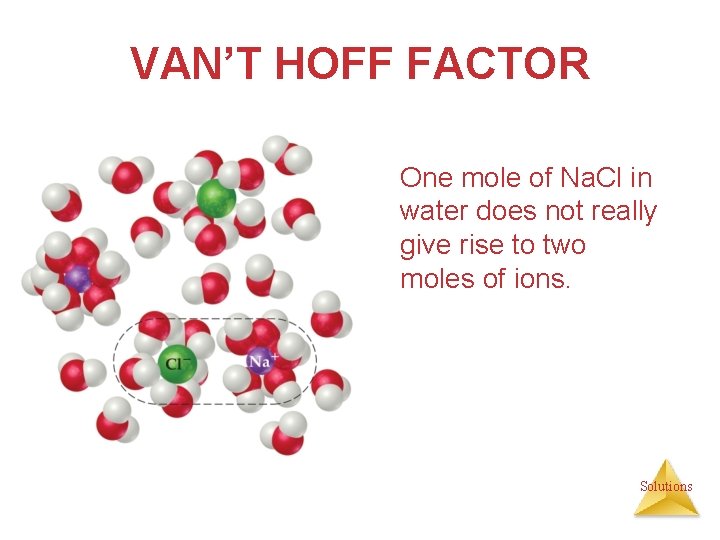
VAN’T HOFF FACTOR One mole of Na. Cl in water does not really give rise to two moles of ions. Solutions

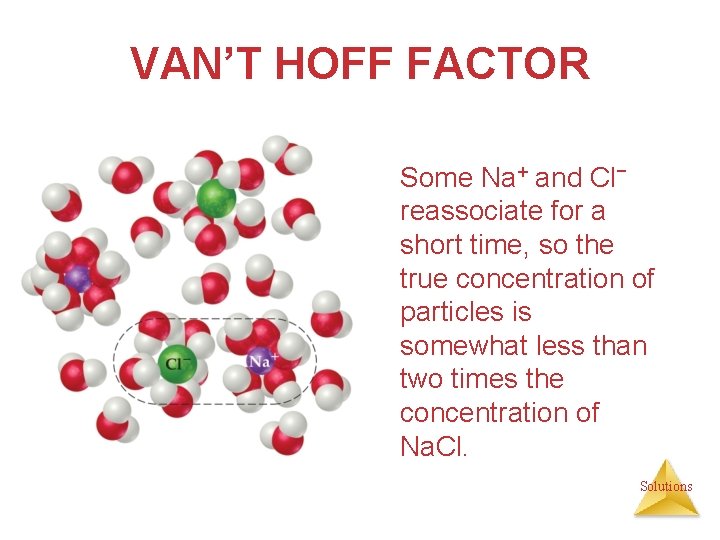
VAN’T HOFF FACTOR Some Na+ and Cl− reassociate for a short time, so the true concentration of particles is somewhat less than two times the concentration of Na. Cl. Solutions

RAOULT’S LAW Solutions

EXAMPLE Calculate the vapor pressure caused by the addition of 100. g of sucrose, C 12 H 22 O 11, to 1000. g of water if the vapor pressure of the pure water at 25 o. C is 23. 8 torr. 100. g sucrose x 1 mol sucrose = 0. 292 mol 342. 30 g sucrose 1000. g water x 1 mole water = 55. 5 mol water 18. 02 g water χ water = 55. 5 mol = 0. 995 0. 292 mol + 55. 5 mol Psoln = 0. 995 × 23. 8 torr = 23. 7 torr Solutions

PRACTICE FIVE Calculate the expected vapor pressure at 25ºC for a solution prepared by dissolving 158. 0 g of common table sugar (sucrose, molar mass = 342. 30 g/mol) in 643. 5 cm 3 of water. At 25ºC, the density of water is 0. 9971 g/cm 3 and the vapor pressure is 23. 76 torr. Solutions

PRACTICE SIX • Predict the vapor pressure of a solution prepared by mixing 35. 0 g solid Na 2 SO 4 • (molar mass = 142. 04 g/mol) with 175 g water at 25°C. The vapor pressure of pure water at 25°C is 23. 76 torr. Solutions

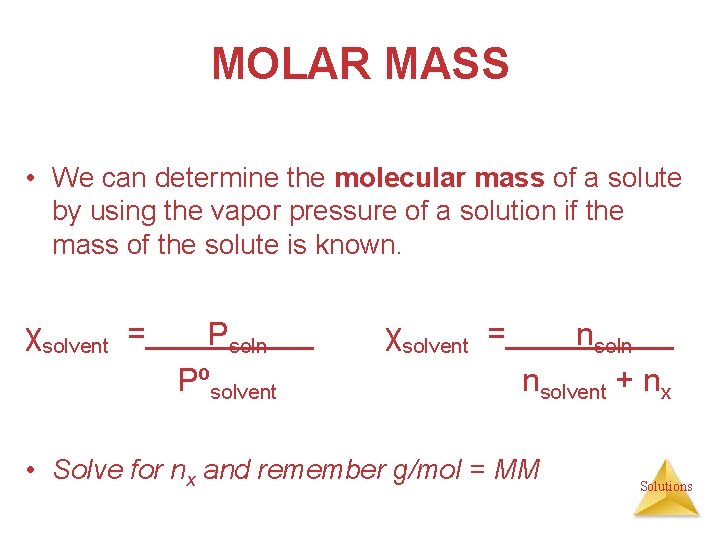
MOLAR MASS • We can determine the molecular mass of a solute by using the vapor pressure of a solution if the mass of the solute is known. χsolvent = Psoln Pºsolvent χsolvent = nsoln nsolvent + nx • Solve for nx and remember g/mol = MM Solutions

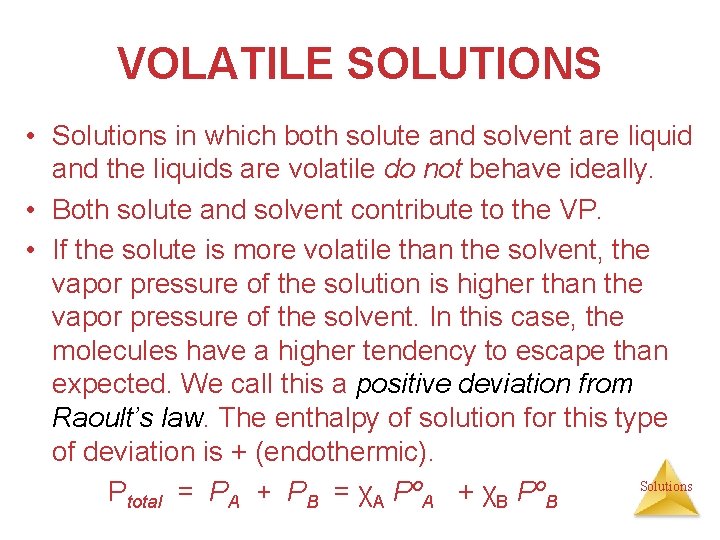
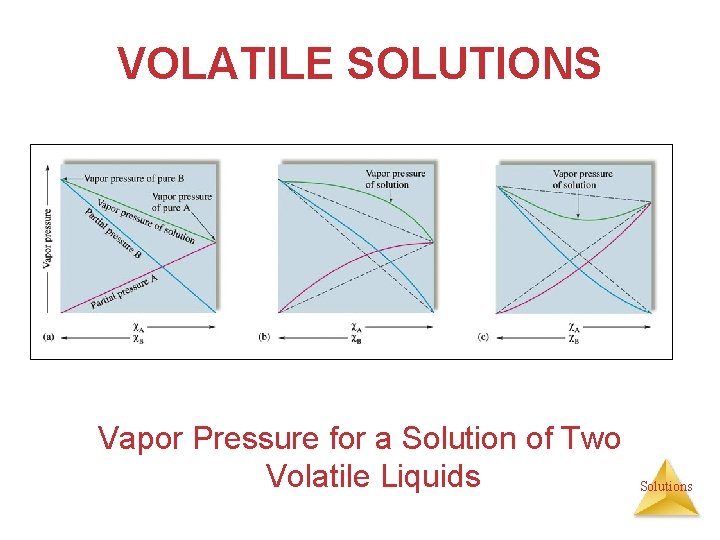
VOLATILE SOLUTIONS • Solutions in which both solute and solvent are liquid and the liquids are volatile do not behave ideally. • Both solute and solvent contribute to the VP. • If the solute is more volatile than the solvent, the vapor pressure of the solution is higher than the vapor pressure of the solvent. In this case, the molecules have a higher tendency to escape than expected. We call this a positive deviation from Raoult’s law. The enthalpy of solution for this type of deviation is + (endothermic). Solutions Ptotal = PA + PB = χA PºA + χB PºB

VOLATILE SOLUTIONS Vapor Pressure for a Solution of Two Volatile Liquids Solutions

PRACTICE SEVEN • A solution is prepared by mixing 5. 81 g acetone (C 3 H 6 O, molar mass = 58. 1 g/mol) and 11. 9 g chloroform (HCCl 3, molar mass = 119. 4 g/mol). At 35°C, this solution has a total vapor pressure of 260. torr. Is this an ideal solution? The vapor pressures of pure acetone and pure chloroform at 35°C are 345 and 293 torr, respectively. Solutions

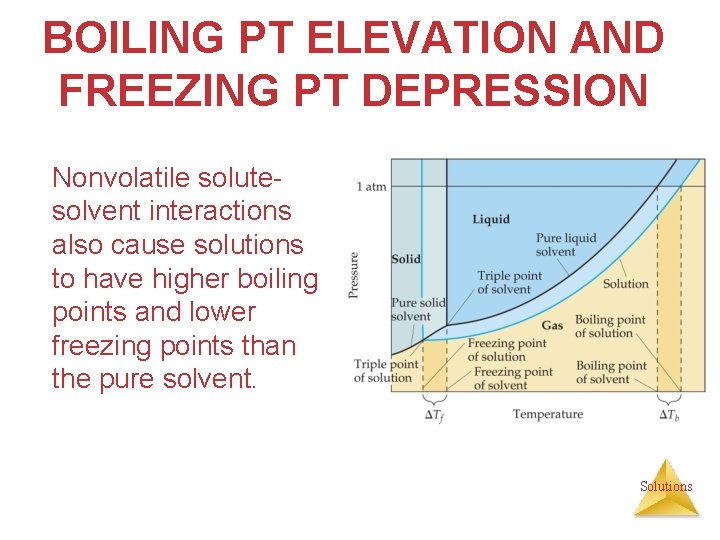
BOILING PT ELEVATION AND FREEZING PT DEPRESSION Nonvolatile solutesolvent interactions also cause solutions to have higher boiling points and lower freezing points than the pure solvent. Solutions

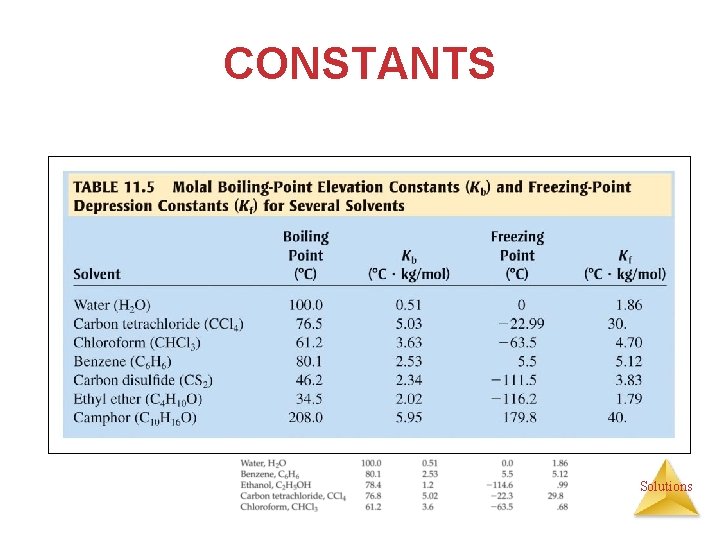
BOILING POINT ELEVATION • Because vapor pressure is lowered by the addition of a nonvolatile solute, more energy must be added to the system to achieve a vapor pressure equal to the atmospheric pressure (definition of normal BP), so the boiling point is increased. • Change in boiling point is proportional to the molality of the solution: Tb = Kb m i § Kb is the molal boiling pt elevation constant, a property of the solvent. § i = van’t Hoff factor Solutions § Tb is added to the normal boiling point of the solvent.

FREEZING PT DEPRESSION • Because vapor pressure is lowered by the addition of a nonvolatile solute, less energy must be added to the system to achieve a temperature at which the vapor pressure of the solid and the liquid are equal (definition of FP or MP). • The change in freezing point can be found similarly: Tf = Kf m I • Kf is the molal freezing point depression constant of the solvent. • Tf is subtracted from the normal freezing pt of the solvent. Solutions

Boiling Point Elevation and Freezing Point Depression Note that in both equations, T does not depend on what the solute is, but only on how many particles are dissolved. Tb = Kb m i Tf = Kf m i Solutions

WHY DEPRESSED? • Molecules cluster in order to freeze. They must be attracted to one another and have a spot in which to cluster. Solute molecules get in the way! • The more ions in solution, the greater the effect on the freezing point and the boiling point. • A solution does not have a sharply defined freezing point, a solvent does. Useful for separation purposes in fractional crystallization. Solutions

CONSTANTS Solutions

PRACTICE EIGHT • Calculate the freezing point and boiling point of a solution of 100. g ethylene glycol (C 2 H 6 O 2) in 900. g of water. Solutions

CALCULATING MOLAR MASS Determining the molar mass (MM) of a solute using freezing-point depression or boilingpoint elevation • solute concentration must be low (0. 10 m) • disadvantage — compound must be nonvolatile and stable at the boiling point. • still used widely • remember that the units you seek are grams/mole! Solutions

PRACTICE NINE • A solution was prepared by dissolving 18. 00 g glucose in 150. 0 g water. The resulting solution was found to have a boiling point of 100. 34ºC. Calculate the molar mass of glucose. Glucose is a molecular solid that is present as individual molecules in solution. Solutions

PRACTICE TEN • What mass of ethylene glycol (C 2 H 6 O 2, molar mass = 62. 1 g/mol), the main component of antifreeze, must be added to 10. 0 L water to produce a solution for use in a car’s radiator that freezes at − 23. 3ºC? Assume the density of water is exactly 1. 00 g/m. L. Solutions

PRACTICE ELEVEN • A chemist is trying to identify a human hormone, which controls metabolism, by determining its molar mass. A sample weighing 0. 546 g was dissolved in 15. 0 g benzene, and the freezing-point depression was determined to be 0. 240ºC. Calculate the molar mass of the hormone. Solutions

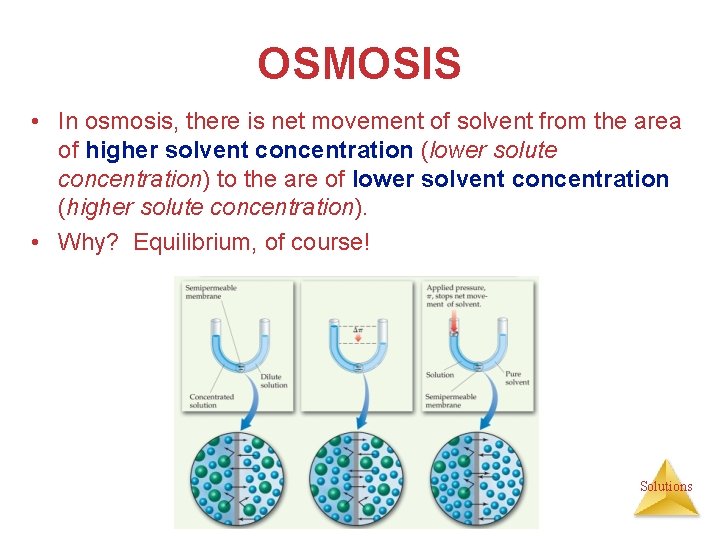
OSMOSIS • Some substances form semipermeable membranes, allowing some smaller particles to pass through, but blocking other larger particles. • In biological systems, most semipermeable membranes allow water to pass through, but solutes are not free to do so. Solutions

OSMOSIS • In osmosis, there is net movement of solvent from the area of higher solvent concentration (lower solute concentration) to the are of lower solvent concentration (higher solute concentration). • Why? Equilibrium, of course! Solutions

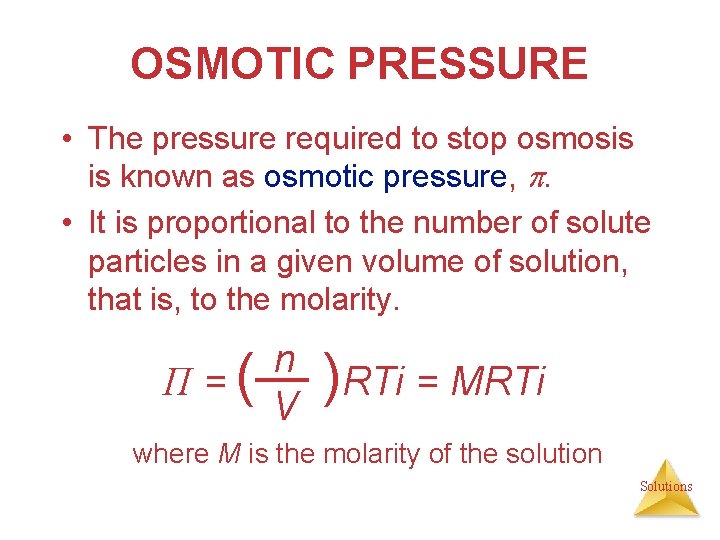
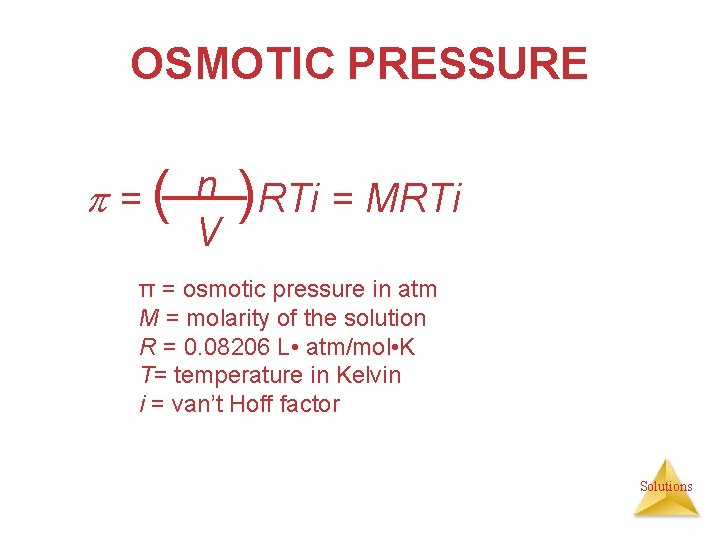
OSMOTIC PRESSURE • The pressure required to stop osmosis is known as osmotic pressure, . • It is proportional to the number of solute particles in a given volume of solution, that is, to the molarity. Π=( n V )RTi = MRTi where M is the molarity of the solution Solutions

OSMOTIC PRESSURE = ( n )RTi = MRTi V π = osmotic pressure in atm M = molarity of the solution R = 0. 08206 L • atm/mol • K T= temperature in Kelvin i = van’t Hoff factor Solutions

OSMOTIC PRESSURE • The use of osmotic pressure calculations for determining the molecular mass of an unknown substance is more accurate than the use of freezing-point depression or boiling point elevation data because a small concentration of solute produces a relatively large osmotic pressure. • Ideal for measuring molar masses of large molecules of biological importance. Solutions

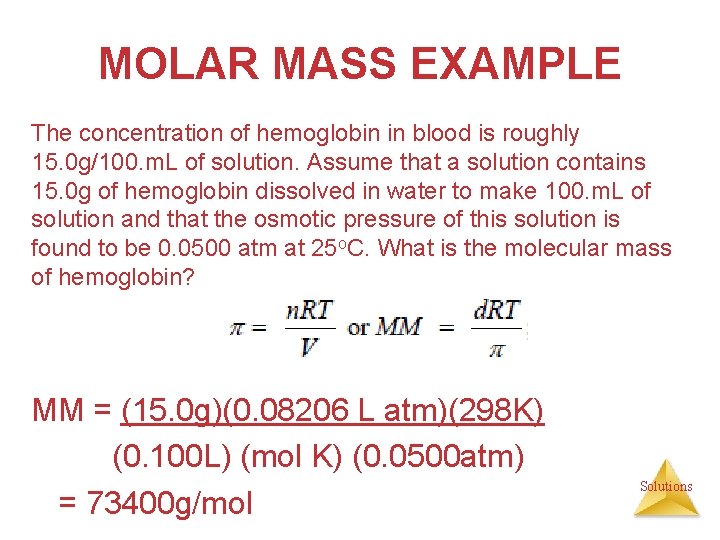
MOLAR MASS EXAMPLE The concentration of hemoglobin in blood is roughly 15. 0 g/100. m. L of solution. Assume that a solution contains 15. 0 g of hemoglobin dissolved in water to make 100. m. L of solution and that the osmotic pressure of this solution is found to be 0. 0500 atm at 25 o. C. What is the molecular mass of hemoglobin? MM = (15. 0 g)(0. 08206 L atm)(298 K) (0. 100 L) (mol K) (0. 0500 atm) = 73400 g/mol Solutions

PRACTICE TWELVE To determine the molar mass of a certain protein, 1. 00 × 10− 3 g of it was dissolved in enough water to make 1. 00 m. L of solution. The osmotic pressure of this solution was found to be 1. 12 torr at 25. 0ºC. Calculate the molar mass of the protein. Solutions

PRACTICE THIRTEEN What concentration of sodium chloride in water is needed to produce an aqueous solution isotonic with blood (π = 7. 70 atm at 25ºC)? Solutions

PRACTICE FOURTEEN The observed osmotic pressure for a 0. 10 M solution of Fe(NH 4)2(SO 4)2 at 25ºC is 10. 8 atm. Compare the expected and experimental values for i. Solutions

APPLICATIONS • Dialysis — a phenomenon in which a semipermeable membrane allows transfer of both solvent molecules and small solute molecules and ions. • Occurs in walls of most plant and animal cells • Kidney dialysis is one of most important applications – waste molecules move into the “wash” solution and filter the blood. Solutions

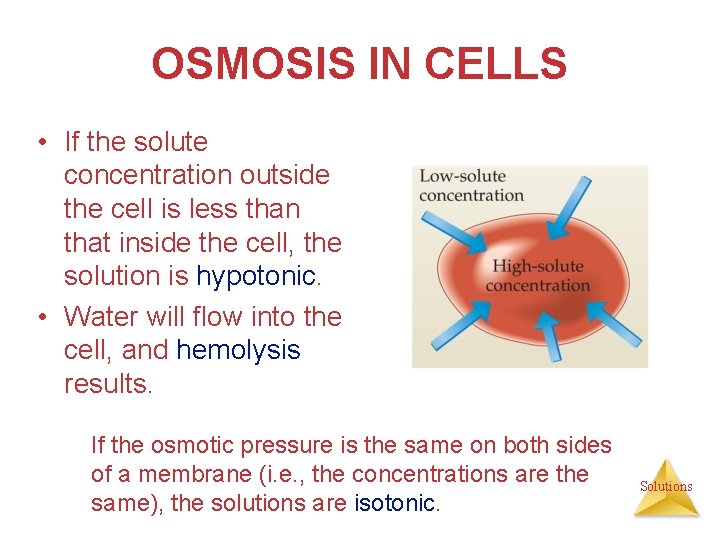
OSMOSIS IN CELLS • If the solute concentration outside the cell is less than that inside the cell, the solution is hypotonic. • Water will flow into the cell, and hemolysis results. If the osmotic pressure is the same on both sides of a membrane (i. e. , the concentrations are the same), the solutions are isotonic. Solutions

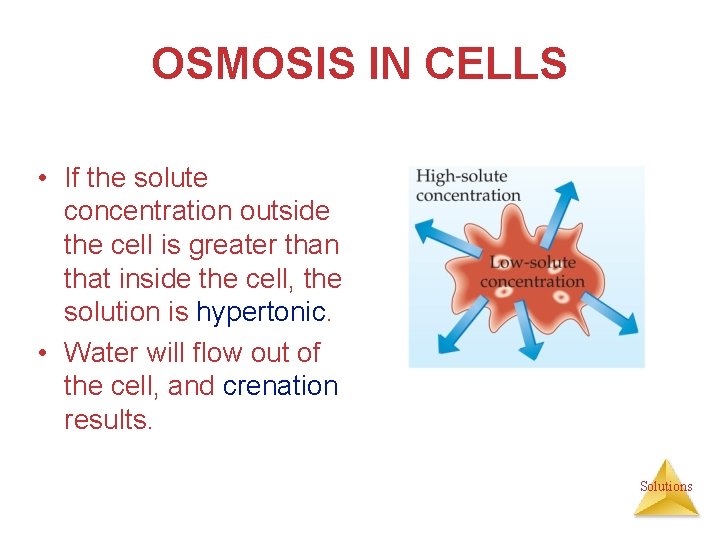
OSMOSIS IN CELLS • If the solute concentration outside the cell is greater than that inside the cell, the solution is hypertonic. • Water will flow out of the cell, and crenation results. Solutions

REVERSE OSMOSIS • reverse osmosis — the process occurring when the high external pressure on a solution causes a net flow of solvent through a semipermeable membrane from the solution to the solvent. • used in desalination (the membrane here acts as a “molecular filter” to remove solute particles). The need for this process will probably increase as the need for drinkable water increases. Solutions

COLLOIDS • Suspensions of particles larger than individual ions or molecules, but too small to be settled out by gravity. • The dispersed colloidal particles are larger than a simple molecule but small enough to remain distributed and not settle out. • A colloidal particle has a diameter between 1 and 1000 nm. • Colloids have an enormous total surface area. • The particles stay suspended because of Solutions electrostatic repulsion.

COLLOIDS • Coagulation, destruction of a colloid, occurs by heating (particles collide so hard that they stick together) or by the addition of an electrolyte (neutralizes ion layers). • Tyndall effect — the scattering of light by particles • Used to distinguish between a suspension and a true solution. A true solution has particles that are too small to scatter light. Solutions

TYNDALL EFFECT Left side exhibits the Tyndall Effect. Right side does not. • Colloidal suspensions can scatter rays of light. • This phenomenon is known as the Tyndall effect. Solutions

COLLOIDS • Brownian motion— a characteristic movement in which the particles change speed and direction erratically (solvent molecules collide with the colloidal particles). • Suspensions are temporary solutions. They will settle eventually—colloids will not do this. • Solutions are permanent. Particles are really small. Colloids lie in between solutions and suspensions! Solutions

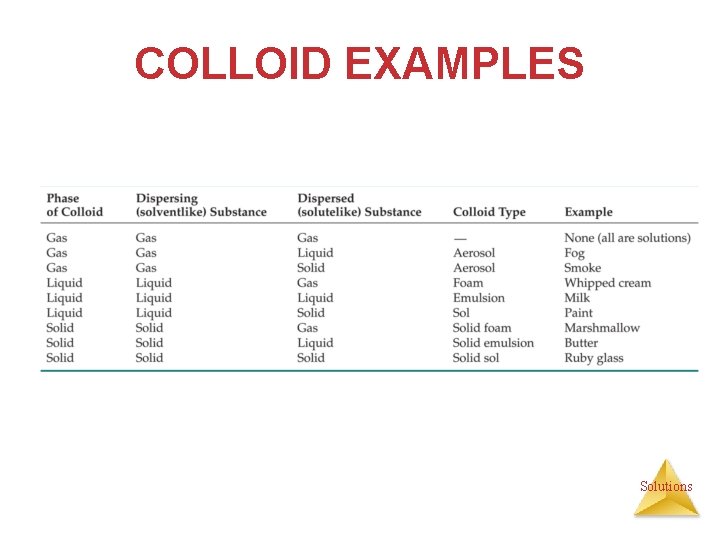
COLLOID EXAMPLES Solutions

COLLOID EXAMPLES • Foam — colloidal dispersion of a gas dispersed in a liquid or solid (ex. whipped cream and marshmallows) • Aerosol — colloidal dispersion of a liquid or solid dispersed in a gas (ex. fog and smoke) • Emulsion — colloidal dispersion of a liquid dispersed in a solid or liquid (ex. butter and milk) • Sol — colloidal dispersion of a solid dispersed in a liquid or solid (ex. paint or ruby). Solutions

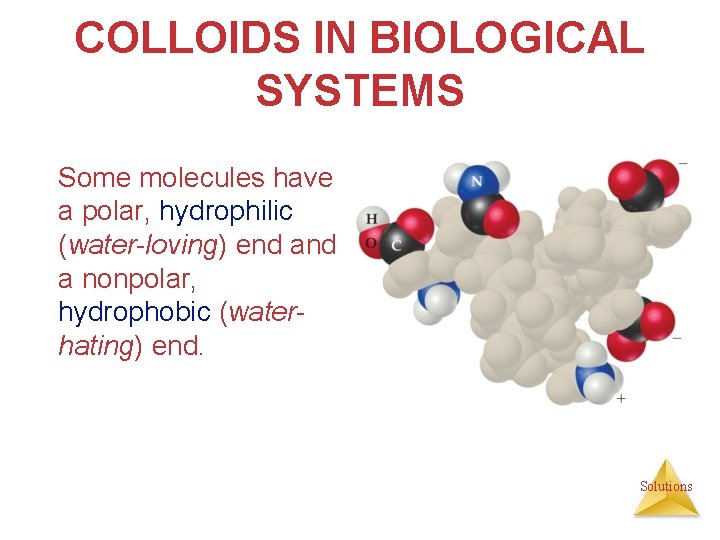
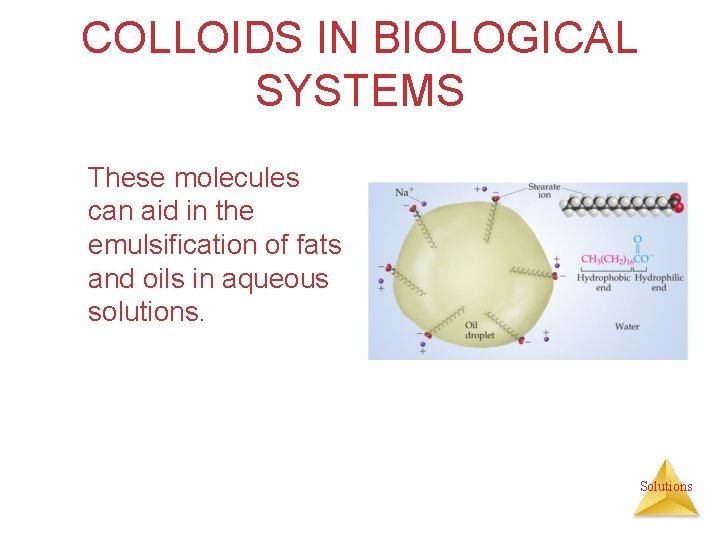
COLLOIDS IN BIOLOGICAL SYSTEMS Some molecules have a polar, hydrophilic (water-loving) end a nonpolar, hydrophobic (waterhating) end. Solutions

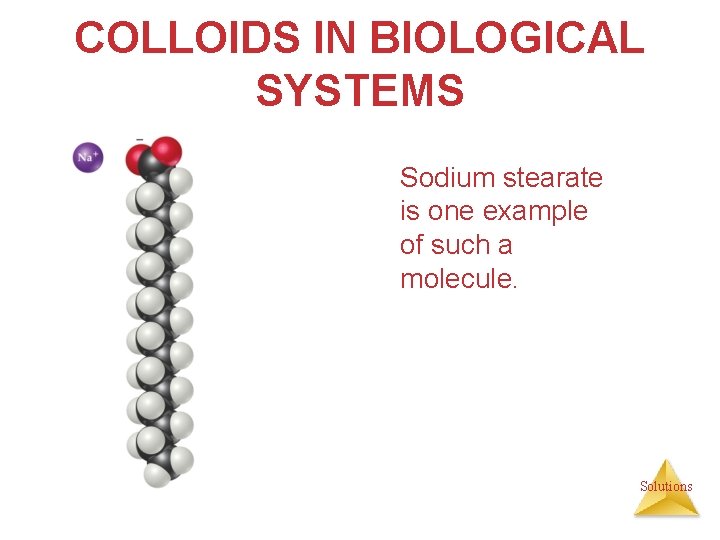
COLLOIDS IN BIOLOGICAL SYSTEMS Sodium stearate is one example of such a molecule. Solutions

COLLOIDS IN BIOLOGICAL SYSTEMS These molecules can aid in the emulsification of fats and oils in aqueous solutions. Solutions