Colligative Properties Colligative Properties physical properties of solutions






- Slides: 8

Colligative Properties

Colligative Properties – physical properties of solutions that are affected by the number of particles (CONCENTRATION) but not by the identity of the dissolved particle (solute) Boiling point elevation Freezing point depression

Ionic vs. Covalent Ionic compounds – dissolve and dissociate into their ions 1 M Na. Cl – 1 M Na+ and 1 M Cl- Creates Covalent 1 M Compounds – dissolve but do not dissociate C 12 H 22 O 12 – 1 M C 12 H 22 O 12 Creates 2 M ions total 1 M dissolved compound For now, we will only worry about COVALENT compounds

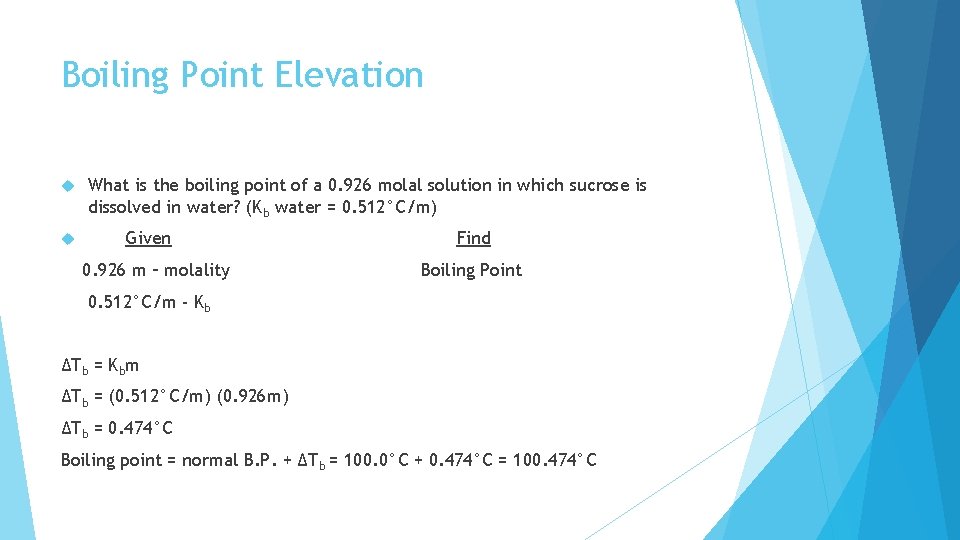
Boiling Point Elevation For a solvent to boil, the vapor pressure = atmospheric pressure Water alone = 100°C Water with sugar = 100. 77°C The boiling point of a solution will always be HIGHER than that of the pure solvent! ΔTb = Kbm ΔTb = Boiling point elevation Kb = Molal boiling point elevation constant (must be given to you!) m = molality

Boiling Point Elevation What is the boiling point of a 0. 926 molal solution in which sucrose is dissolved in water? (Kb water = 0. 512°C/m) Given 0. 926 m – molality Find Boiling Point 0. 512°C/m - Kb ΔTb = Kbm ΔTb = (0. 512°C/m) (0. 926 m) ΔTb = 0. 474°C Boiling point = normal B. P. + ΔTb = 100. 0°C + 0. 474°C = 100. 474°C

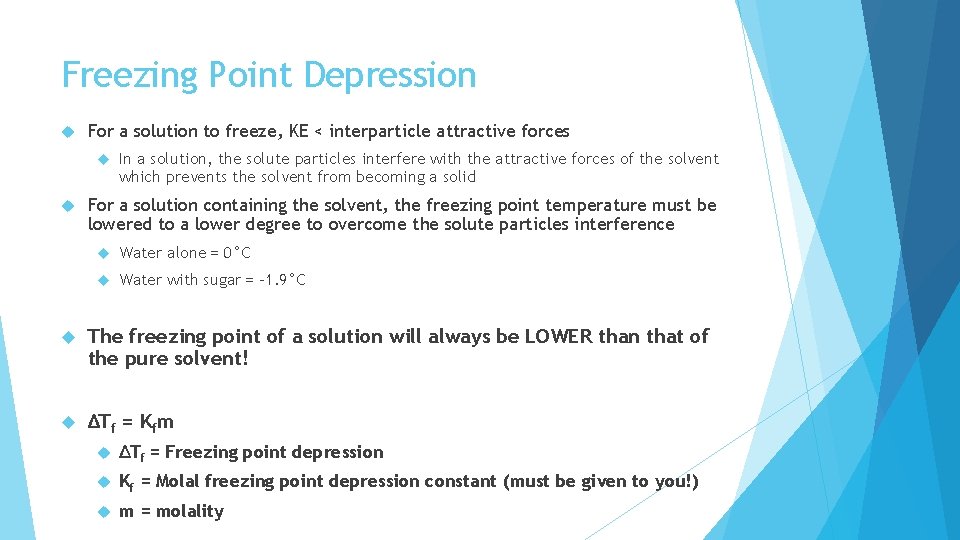
Freezing Point Depression For a solution to freeze, KE < interparticle attractive forces In a solution, the solute particles interfere with the attractive forces of the solvent which prevents the solvent from becoming a solid For a solution containing the solvent, the freezing point temperature must be lowered to a lower degree to overcome the solute particles interference Water alone = 0°C Water with sugar = -1. 9°C The freezing point of a solution will always be LOWER than that of the pure solvent! ΔTf = Kfm ΔTf = Freezing point depression Kf = Molal freezing point depression constant (must be given to you!) m = molality

Freezing Point Depression What is the freezing point of a 1. 57 molal solution in which sucrose is dissolved in water? (Kf water = 1. 858°C/m) Given Find 1. 57 m – molality Freezing Point 1. 858°C/m - Kf ΔTf = Kfm ΔTf = (1. 858°C/m) (1. 57 m) ΔTf = 2. 92°C Freezing point = normal F. P. - ΔTf = 0. 0°C – 2. 92°C = -2. 92°C

Solution Concentration As the concentration (molality) of a solution increases: The boiling point INCREASES The freezing point DECREASES