Colligative Properties Colligative Properties q Colligative properties depend






- Slides: 20

Colligative Properties

Colligative Properties q. Colligative properties depend on quantity of solute molecules. q. Vapor pressure lowering q. Boiling point elevation q. Freezing point depression q. Osmotic pressure

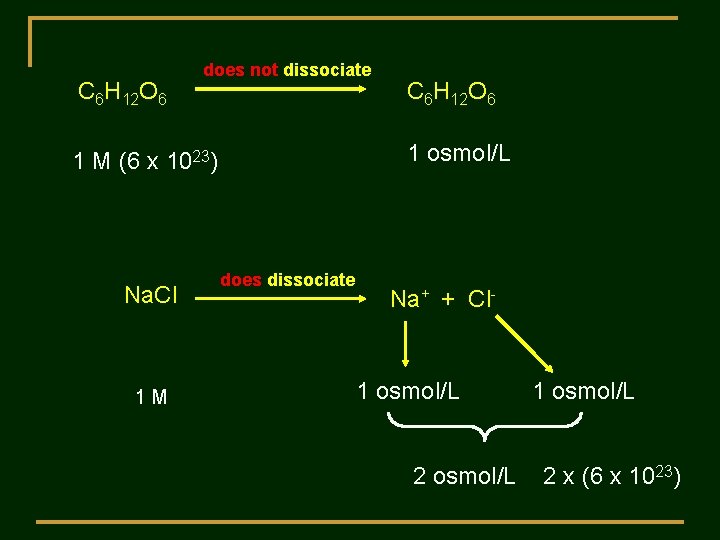
Colligative Properties of Solutions Definition: Properties, that depend on the NUMBER of solute particles present in solution Concentration: Osmol per liter or osmolarity An OSMOL is a mole of solute particle

C 6 H 12 O 6 does not dissociate 1 osmol/L 1 M (6 x 1023) Na. Cl 1 M C 6 H 12 O 6 does dissociate Na+ + Cl- 1 osmol/L 2 osmol/L 1 osmol/L 2 x (6 x 1023)

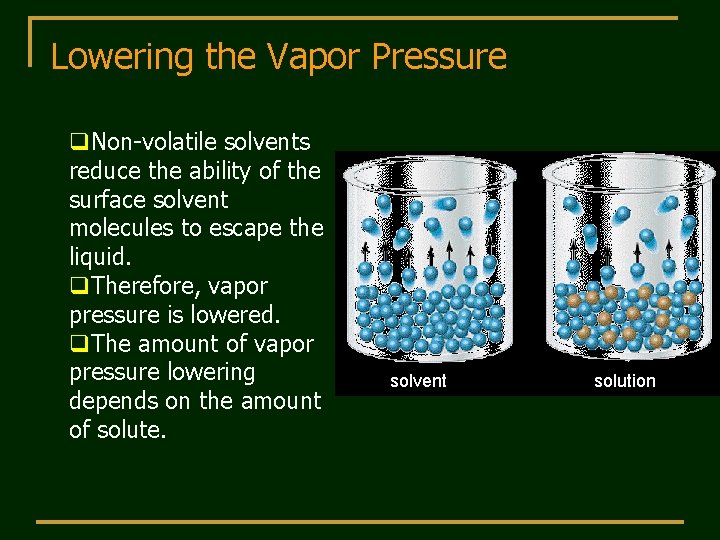
Lowering the Vapor Pressure q. Non-volatile solvents reduce the ability of the surface solvent molecules to escape the liquid. q. Therefore, vapor pressure is lowered. q. The amount of vapor pressure lowering depends on the amount of solute. solvent solution

Vapor pressure lowering q. Raoult’s Law – a nonvolatile solute will lower the vapor pressure of a solvent q. Pure water will have a higher vapor pressure than salt water. q. Ideal solution: one that obeys Raoult’s law. q. Raoult’s law breaks down when the solvent-solvent and solute-solute intermolecular forces are greater than solute-solvent intermolecular forces.

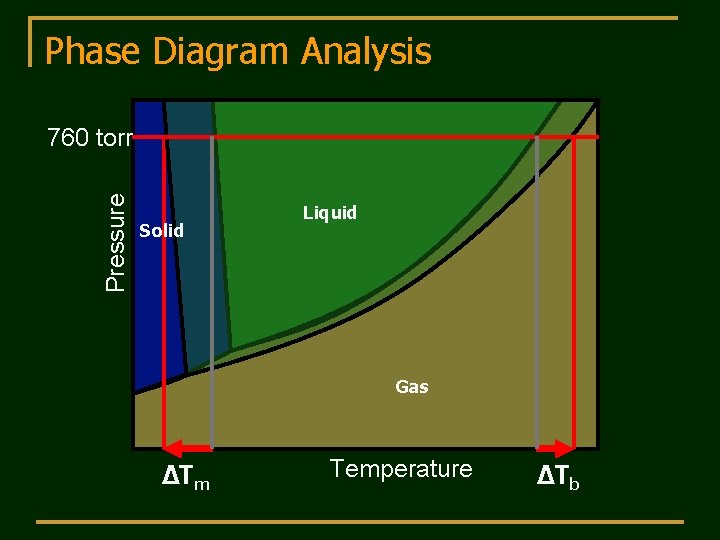
Phase Diagram Analysis Pressure 760 torr Solid Liquid Gas ΔTm Temperature ΔTb

Boiling-Point Elevation Interpret the phase diagram for a solution. Non-volatile solute lowers the vapor pressure. At 1 atm (normal bp of pure liquid) there is a lower vapor pressure of the solution. a higher bp is required to reach a vapor pressure of 1 atm for the solution. ΔTb = Kbmi

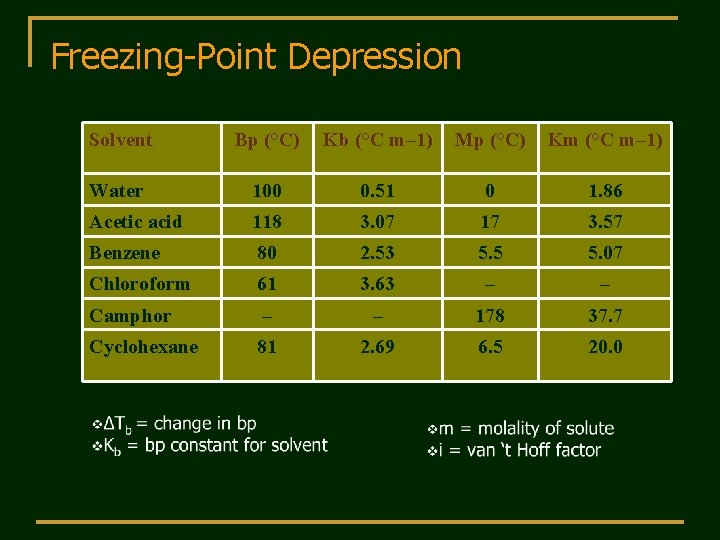
Freezing-Point Depression Solvent Bp (°C) Kb (°C m– 1) Mp (°C) Km (°C m– 1) Water 100 0. 51 0 1. 86 Acetic acid 118 3. 07 17 3. 57 Benzene 80 2. 53 5. 5 5. 07 Chloroform 61 3. 63 – – Camphor – – 178 37. 7 Cyclohexane 81 2. 69 6. 5 20. 0

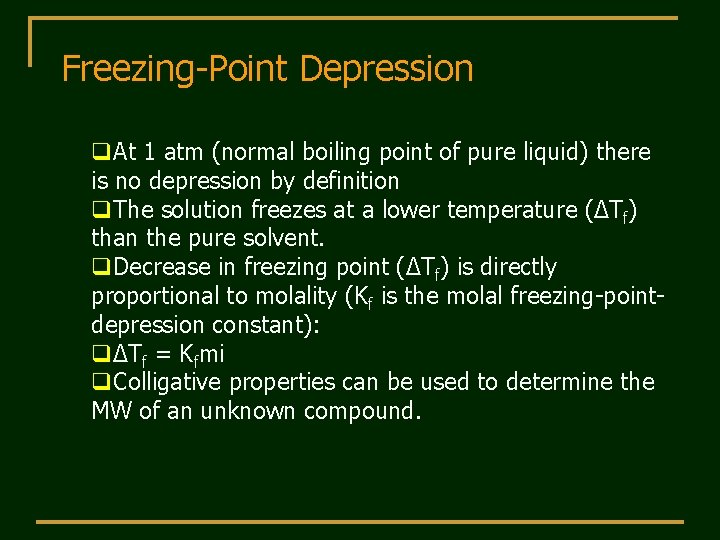
Freezing-Point Depression q. At 1 atm (normal boiling point of pure liquid) there is no depression by definition q. The solution freezes at a lower temperature (ΔTf) than the pure solvent. q. Decrease in freezing point (ΔTf) is directly proportional to molality (Kf is the molal freezing-pointdepression constant): qΔTf = Kfmi q. Colligative properties can be used to determine the MW of an unknown compound.

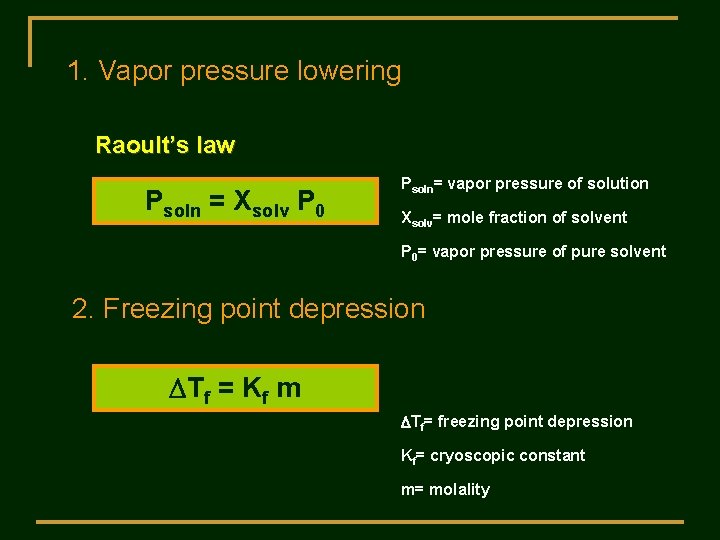
1. Vapor pressure lowering Raoult’s law Psoln = Xsolv P 0 Psoln= vapor pressure of solution Xsolv= mole fraction of solvent P 0= vapor pressure of pure solvent 2. Freezing point depression DTf = Kf m DTf= freezing point depression Kf= cryoscopic constant m= molality

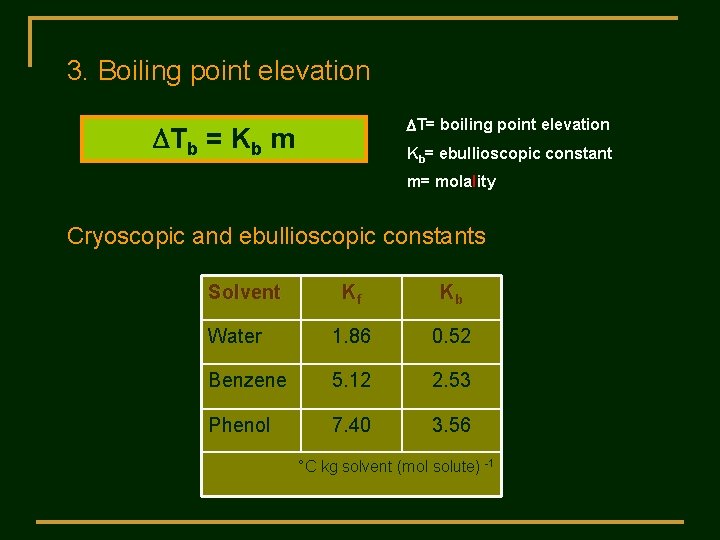
3. Boiling point elevation DT= boiling point elevation DTb = Kb m Kb= ebullioscopic constant m= molality Cryoscopic and ebullioscopic constants Solvent Kf Kb Water 1. 86 0. 52 Benzene 5. 12 2. 53 Phenol 7. 40 3. 56 °C kg solvent (mol solute) -1

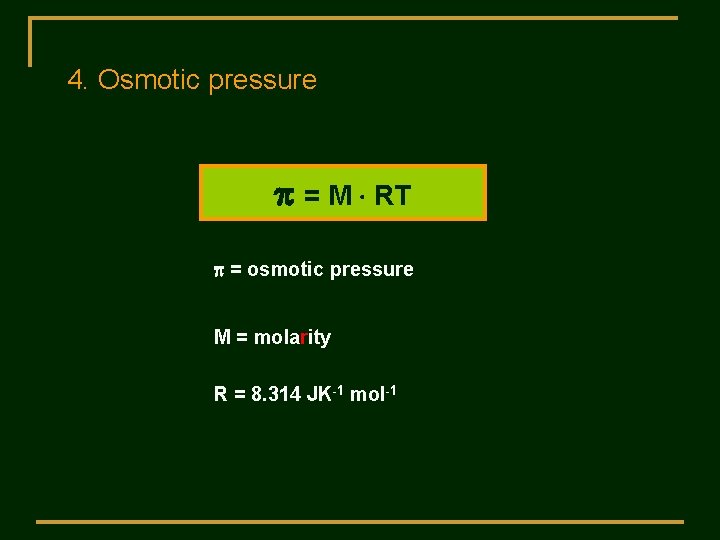
4. Osmotic pressure p = M RT p = osmotic pressure M = molarity R = 8. 314 JK-1 mol-1

Osmotic Pressure q. Semipermeable membrane: permits passage of some components of a solution. Example: cell membranes and cellophane. q. Osmosis: the movement of a solvent from low solute concentration to high solute concentration. q. There is movement in both directions across a semipermeable membrane. q. As solvent moves across the membrane, the fluid levels in the arms becomes uneven. q. Eventually the pressure difference between the arms stops osmosis. Dilute solution Concentrated solution Membrane

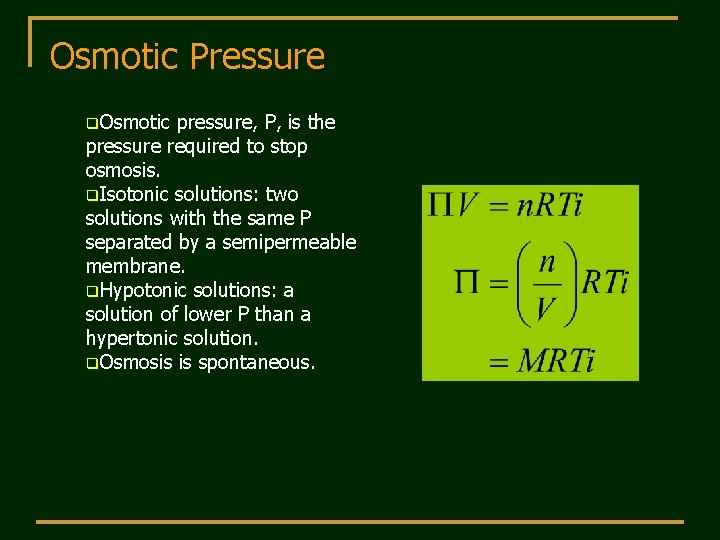
Osmotic Pressure q. Osmotic pressure, P, is the pressure required to stop osmosis. q. Isotonic solutions: two solutions with the same P separated by a semipermeable membrane. q. Hypotonic solutions: a solution of lower P than a hypertonic solution. q. Osmosis is spontaneous.

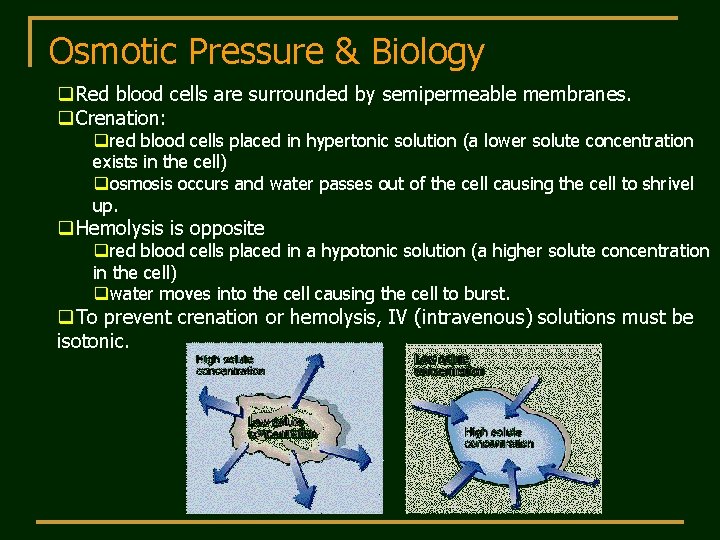
Osmotic Pressure & Biology q. Red blood cells are surrounded by semipermeable membranes. q. Crenation: qred blood cells placed in hypertonic solution (a lower solute concentration exists in the cell) qosmosis occurs and water passes out of the cell causing the cell to shrivel up. q. Hemolysis is opposite qred blood cells placed in a hypotonic solution (a higher solute concentration in the cell) qwater moves into the cell causing the cell to burst. q. To prevent crenation or hemolysis, IV (intravenous) solutions must be isotonic.

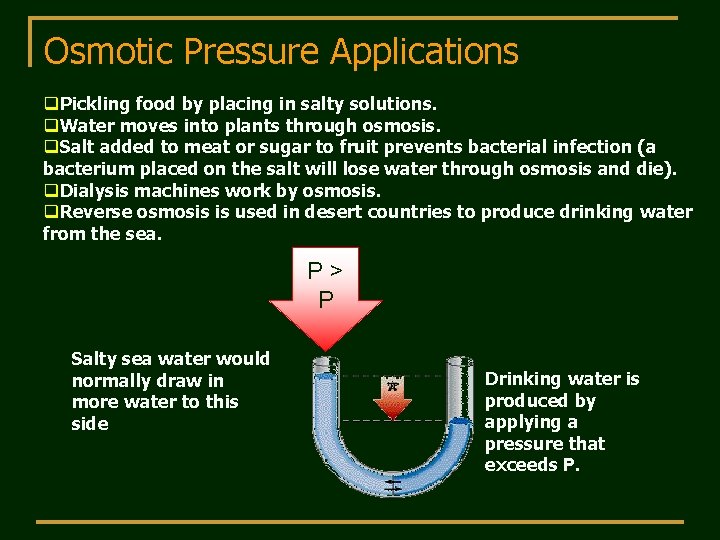
Osmotic Pressure Applications q. Pickling food by placing in salty solutions. q. Water moves into plants through osmosis. q. Salt added to meat or sugar to fruit prevents bacterial infection (a bacterium placed on the salt will lose water through osmosis and die). q. Dialysis machines work by osmosis. q. Reverse osmosis is used in desert countries to produce drinking water from the sea. P> P Salty sea water would normally draw in more water to this side Drinking water is produced by applying a pressure that exceeds P.

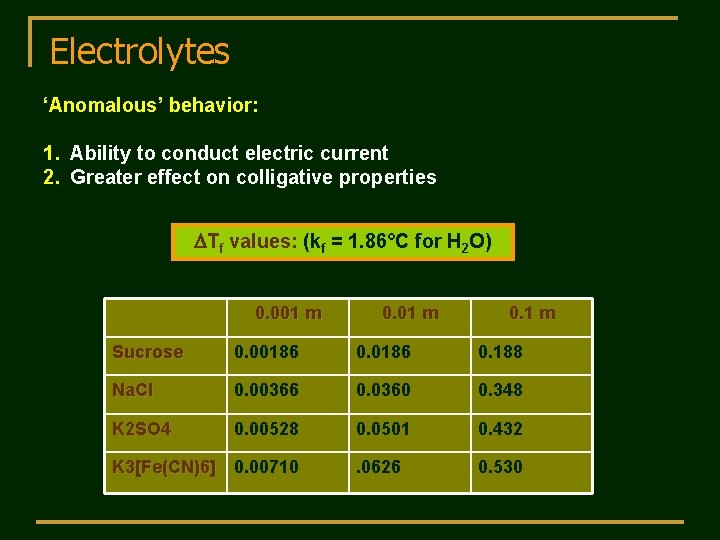
Electrolytes ‘Anomalous’ behavior: 1. Ability to conduct electric current 2. Greater effect on colligative properties DTf values: (kf = 1. 86°C for H 2 O) 0. 001 m 0. 1 m Sucrose 0. 00186 0. 188 Na. Cl 0. 00366 0. 0360 0. 348 K 2 SO 4 0. 00528 0. 0501 0. 432 . 0626 0. 530 K 3[Fe(CN)6] 0. 00710

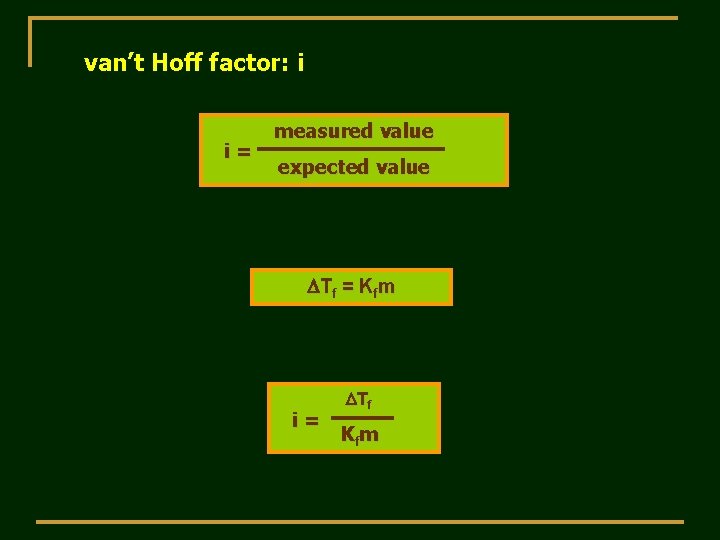
van’t Hoff factor: i i= measured value expected value DTf = Kfm i= DTf Kfm

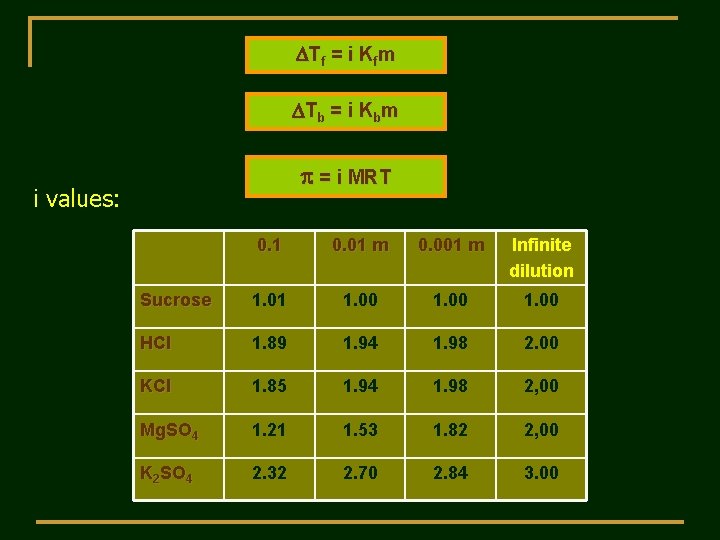
DTf = i Kfm D T b = i Kb m p = i MRT i values: 0. 1 0. 01 m 0. 001 m Infinite dilution Sucrose 1. 01 1. 00 HCl 1. 89 1. 94 1. 98 2. 00 KCl 1. 85 1. 94 1. 98 2, 00 Mg. SO 4 1. 21 1. 53 1. 82 2, 00 K 2 SO 4 2. 32 2. 70 2. 84 3. 00