Colligative Properties of Solutions chapter 16 Colligative properties





- Slides: 13

Colligative Properties of Solutions (chapter 16) Colligative properties = physical properties of solutions that depend on the # of particles dissolved, not the kind of particle.

Colligative Properties n n Lowering vapor pressure Raising boiling point Lowering freezing point Generating an osmotic pressure

2 to focus on… n n Lowering vapor pressure Raising boiling point Lowering freezing point Generating an osmotic pressure

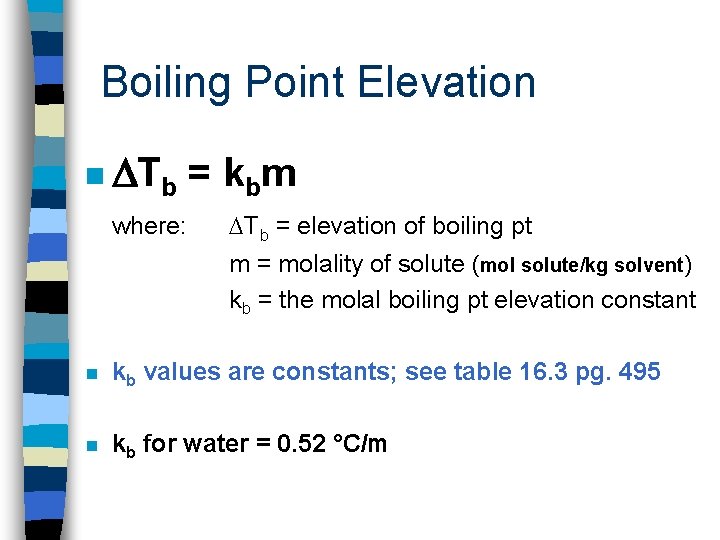
Boiling Point Elevation n a solution that contains a nonvolatile solute has a higher boiling pt than the pure solvent; the boiling pt elevation is proportional to the # of moles of solute dissolved in a given mass of solvent. Like when adding salt to a pot of boiling water to make pasta

Boiling Point Elevation n Tb where: = k bm Tb = elevation of boiling pt m = molality of solute (mol solute/kg solvent) kb = the molal boiling pt elevation constant n kb values are constants; see table 16. 3 pg. 495 n kb for water = 0. 52 °C/m

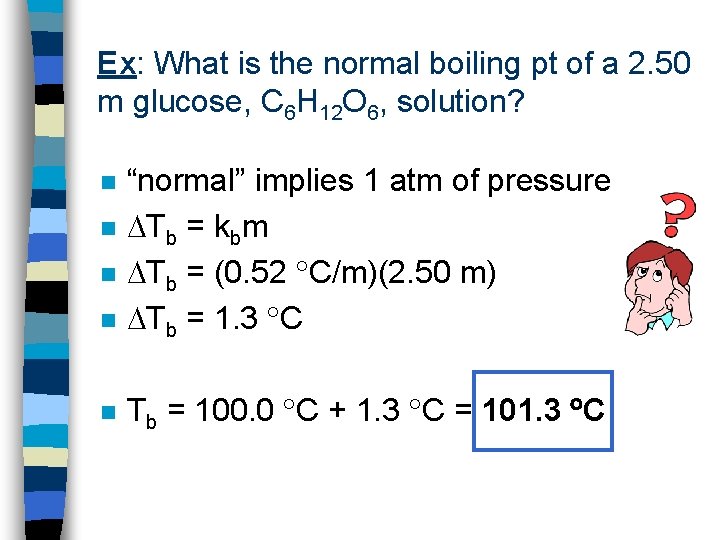
Ex: What is the normal boiling pt of a 2. 50 m glucose, C 6 H 12 O 6, solution? n “normal” implies 1 atm of pressure Tb = kbm Tb = (0. 52 C/m)(2. 50 m) Tb = 1. 3 C n Tb = 100. 0 C + 1. 3 C = 101. 3 C n n n

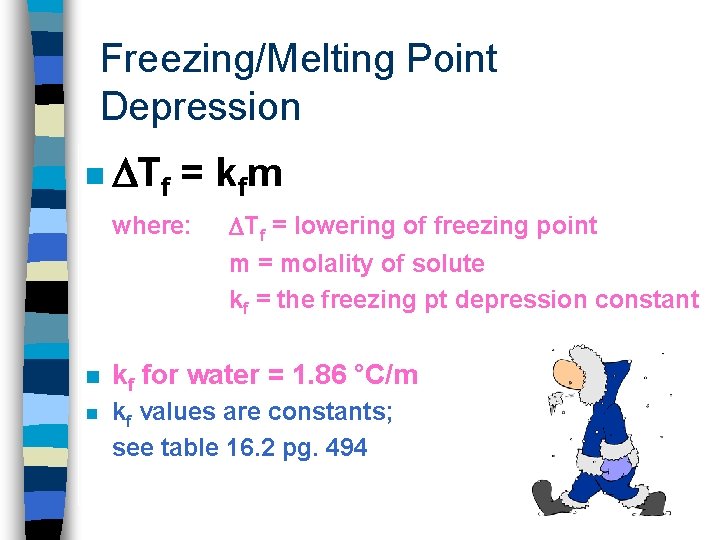
Freezing/Melting Point Depression n The freezing point of a solution is always lower than that of the pure solvent. Like when salting roads in snowy places so the roads don’t ice over or when making ice cream

Freezing/Melting Point Depression n Tf = k fm where: Tf = lowering of freezing point m = molality of solute kf = the freezing pt depression constant n kf for water = 1. 86 °C/m n kf values are constants; see table 16. 2 pg. 494

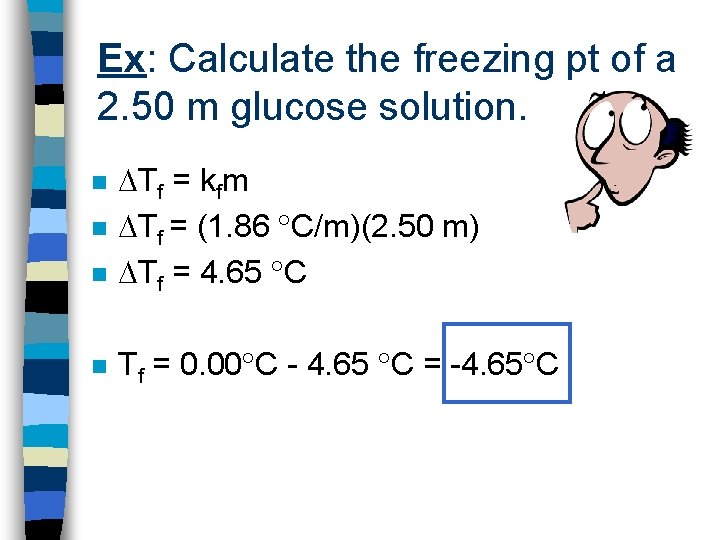
Ex: Calculate the freezing pt of a 2. 50 m glucose solution. n Tf = kfm Tf = (1. 86 C/m)(2. 50 m) Tf = 4. 65 C n Tf = 0. 00 C - 4. 65 C = -4. 65 C n n

Electrolytes and Colligative Properties • Colligative properties depend on the # of particles present in solution. • Because ionic solutes dissociate into ions, they have a greater effect on freezing pt and boiling pt than molecular solids of the same molal conc.

Electrolytes and Colligative Properties n For example, the freezing pt of water is lowered by 1. 86°C with the addition of any molecular solute at a concentration of 1 m. – Such as C 6 H 12 O 6, or any other covalent compound n However, a 1 m Na. Cl solution contains 2 molal conc. of IONS. Thus, the freezing pt depression for Na. Cl is 3. 72°C…double that of a molecular solute. – Na. Cl Na+ + Cl- (2 particles)

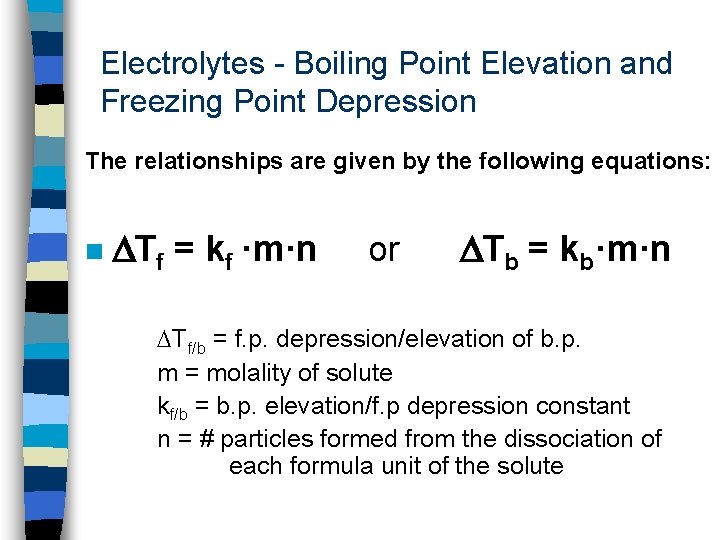
Electrolytes - Boiling Point Elevation and Freezing Point Depression The relationships are given by the following equations: n Tf = kf ·m·n or Tb = kb·m·n Tf/b = f. p. depression/elevation of b. p. m = molality of solute kf/b = b. p. elevation/f. p depression constant n = # particles formed from the dissociation of each formula unit of the solute

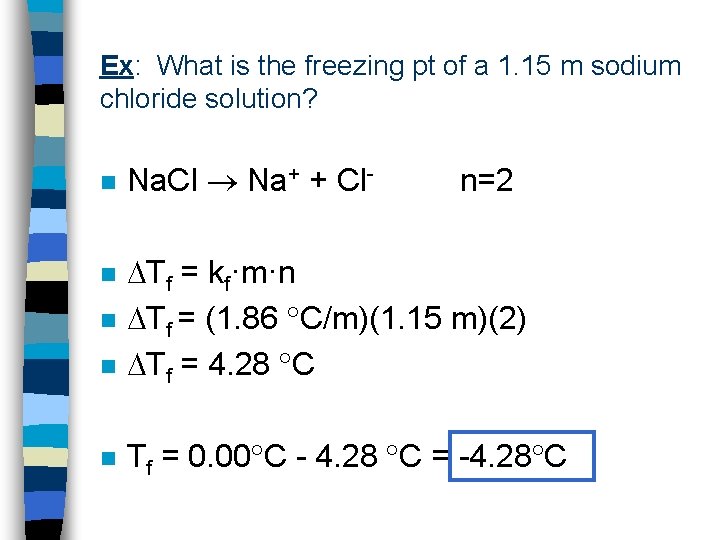
Ex: What is the freezing pt of a 1. 15 m sodium chloride solution? n Na. Cl Na+ + Cl- n=2 n Tf = kf·m·n Tf = (1. 86 C/m)(1. 15 m)(2) Tf = 4. 28 C n Tf = 0. 00 C - 4. 28 C = -4. 28 C n n