Chapter 15 Solutions Solution homogeneous mixture of two


























- Slides: 48

Chapter 15 - Solutions

Ø Solution – homogeneous mixture of two or more substances in the same physical state 1. solute – substance being dissolved l Ex. salt, sugar solvent – material in which solute is dissolved 2. l Ex. water is the universal solvent

Salt water

Salt water There is more solvent than solute in a solution. Ø Ex. more water than salt Ø

Soluble – able to be dissolved Ø Ex. salt in water Ø Insoluble – does not dissolve Ø Ex. oil in water Ø

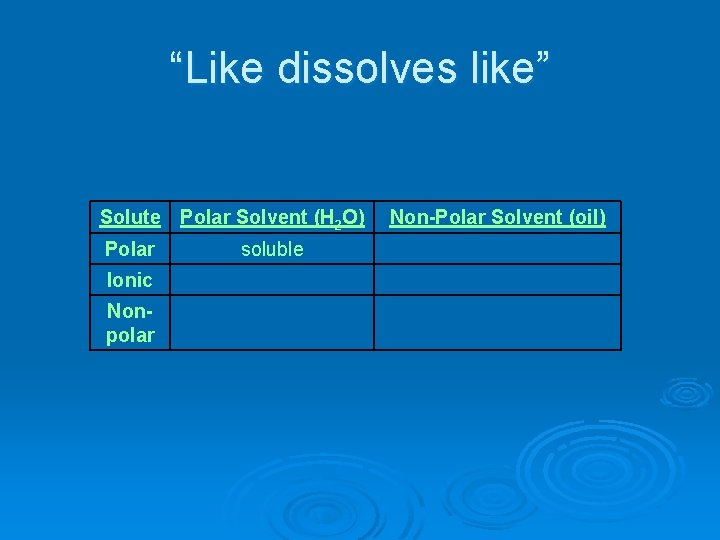
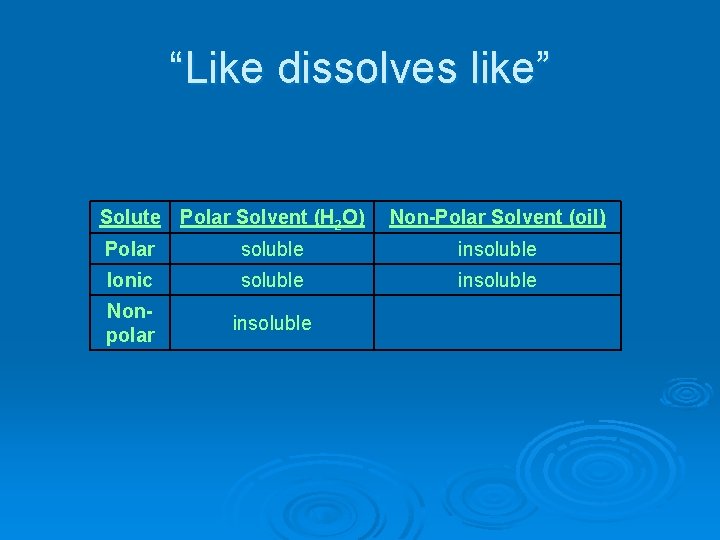
“Like dissolves like” Ø Polar solutes will dissolve in polar solvents Ø Nonpolar solutes will dissolve in nonpolar solvents

“Like dissolves like” Solute Polar Solvent (H 2 O) Polar Ionic Nonpolar soluble Non-Polar Solvent (oil)

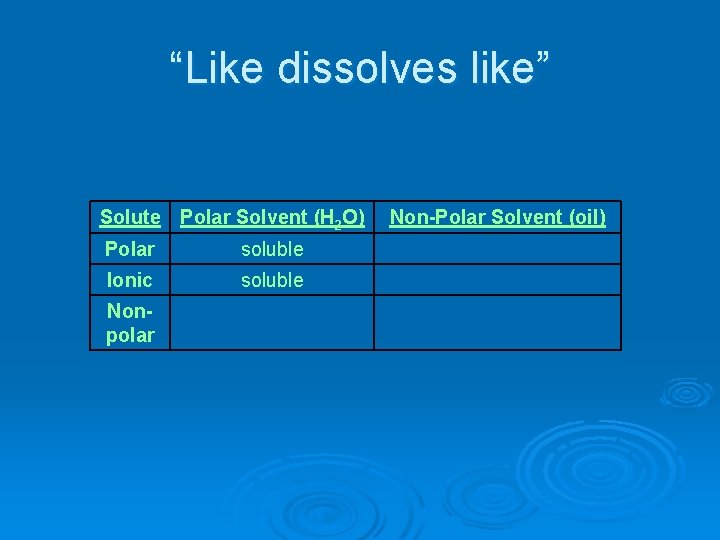
“Like dissolves like” Solute Polar Solvent (H 2 O) Polar soluble Ionic soluble Nonpolar Non-Polar Solvent (oil)

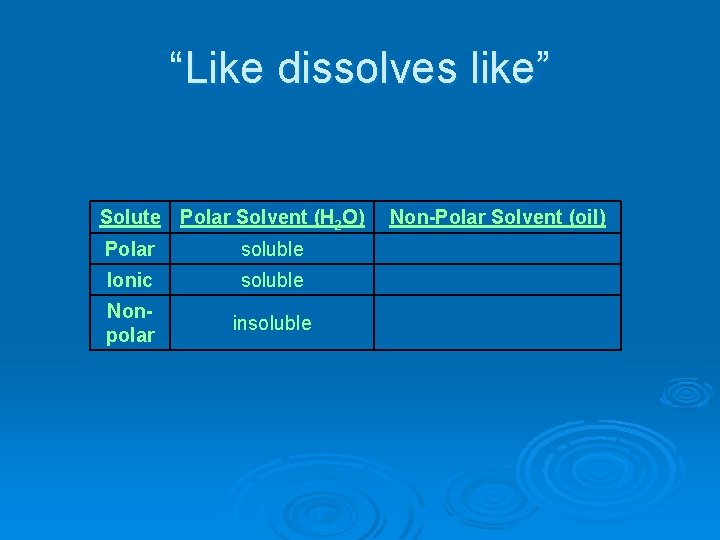
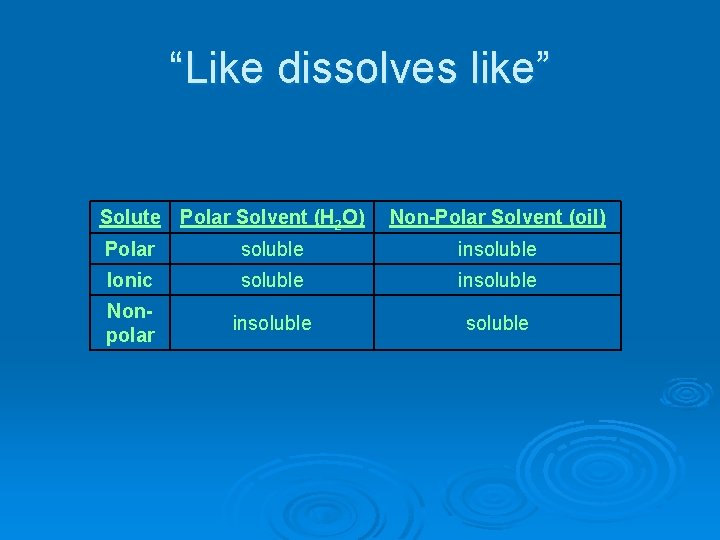
“Like dissolves like” Solute Polar Solvent (H 2 O) Polar soluble Ionic soluble Nonpolar insoluble Non-Polar Solvent (oil)

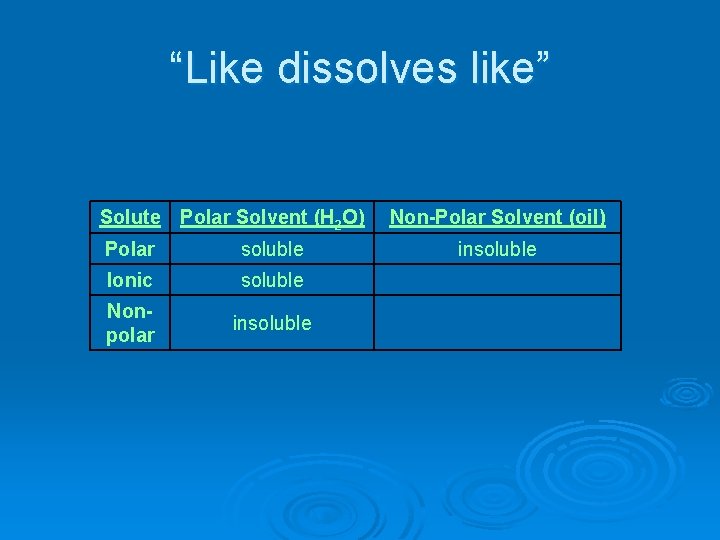
“Like dissolves like” Solute Polar Solvent (H 2 O) Polar soluble Ionic soluble Nonpolar insoluble Non-Polar Solvent (oil) insoluble

“Like dissolves like” Solute Polar Solvent (H 2 O) Non-Polar Solvent (oil) Polar soluble insoluble Ionic soluble insoluble Nonpolar insoluble

“Like dissolves like” Solute Polar Solvent (H 2 O) Non-Polar Solvent (oil) Polar soluble insoluble Ionic soluble insoluble Nonpolar insoluble

Soluble – able to be dissolved Ø Ex. salt in water Ø Ionic dissolves in polar water. Insolube – does not dissolve Ø Ex. oil in water Ø Nonpolar oil does not dissolve in polar water.

Oil Spills

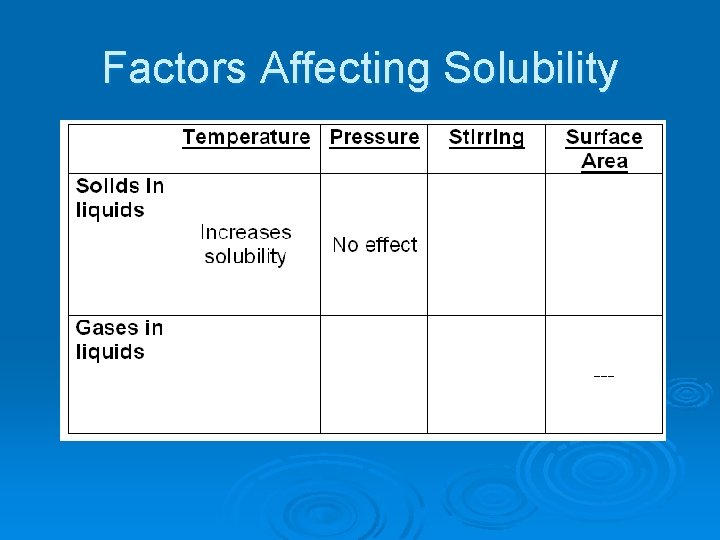
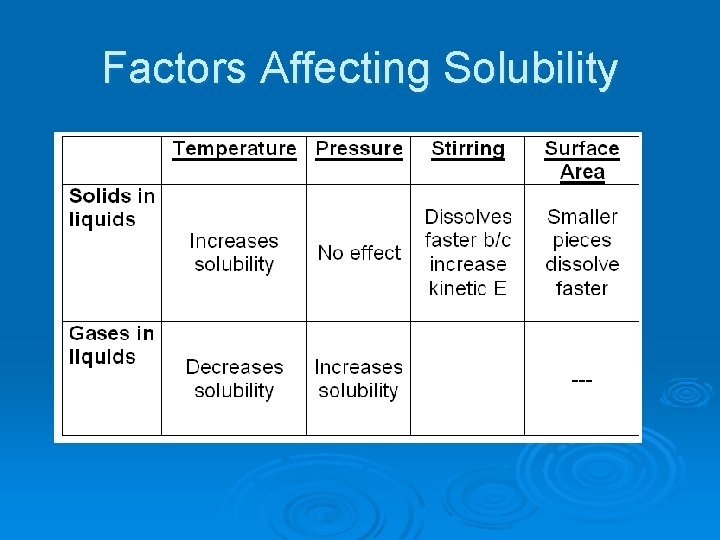
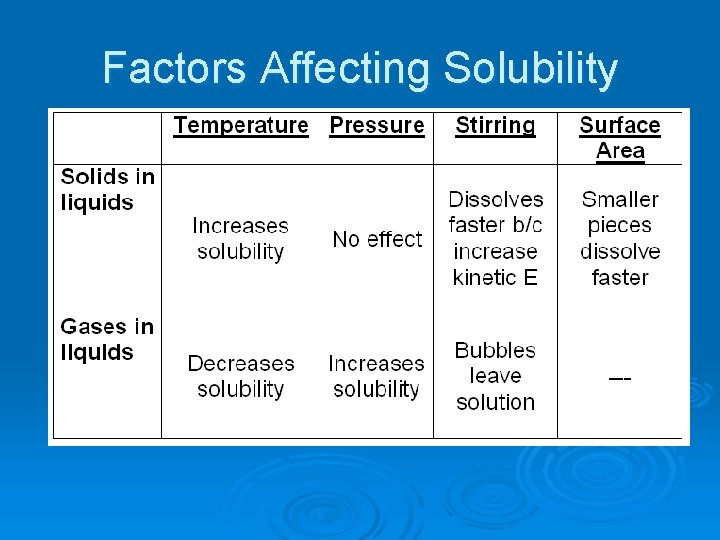
Factors Affecting Solubility

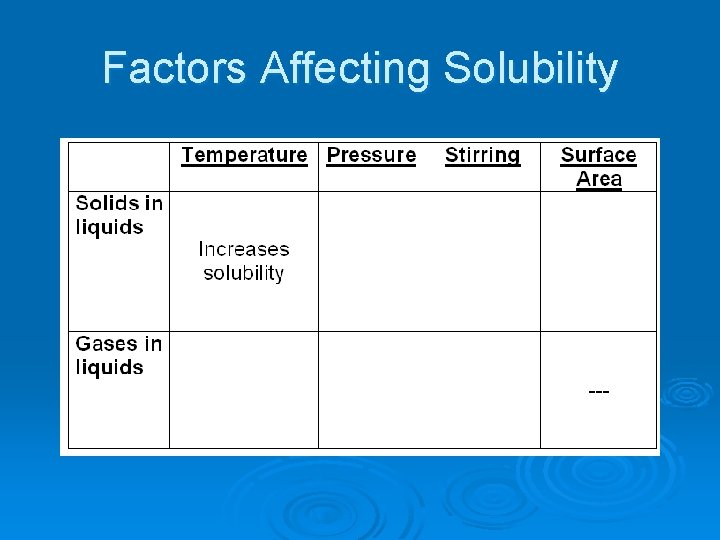
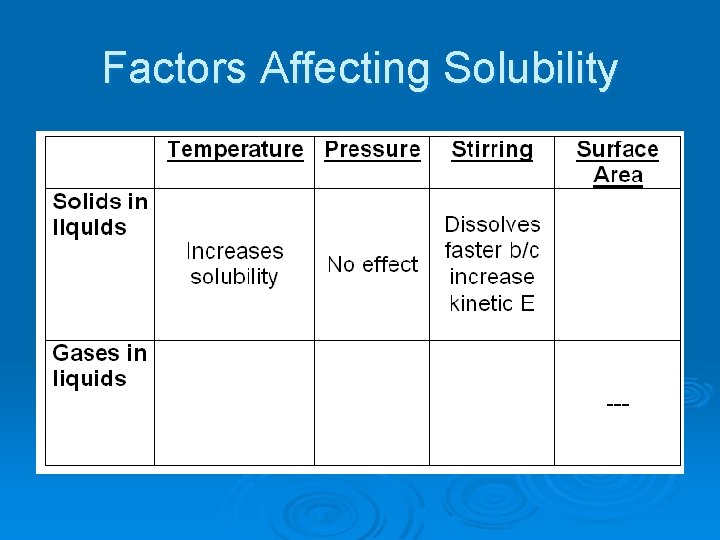
Factors Affecting Solubility

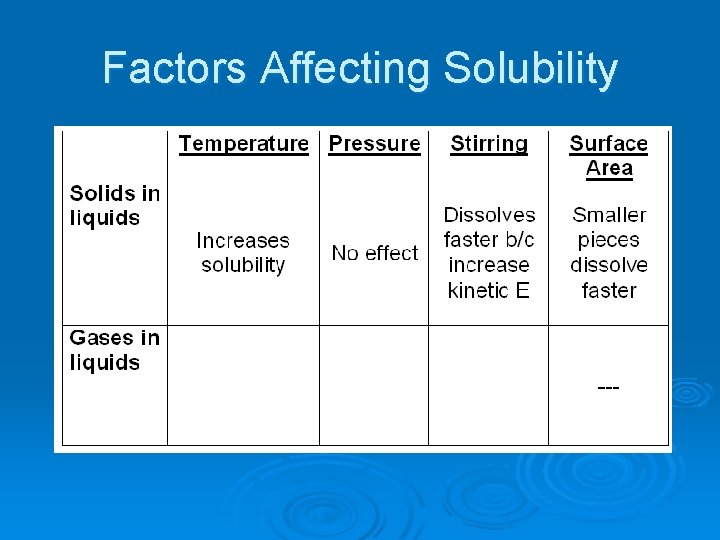
Factors Affecting Solubility

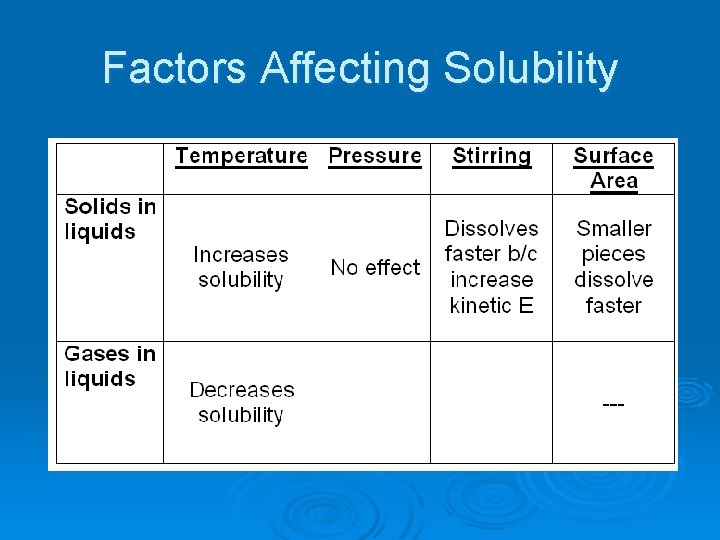
Factors Affecting Solubility

Factors Affecting Solubility

Factors Affecting Solubility

Factors Affecting Solubility

Factors Affecting Solubility

Dissolved Oxygen

Thermal Pollution


Algae Blooms

Aqueous (aq) Ø When a material is dissolved in water vs.

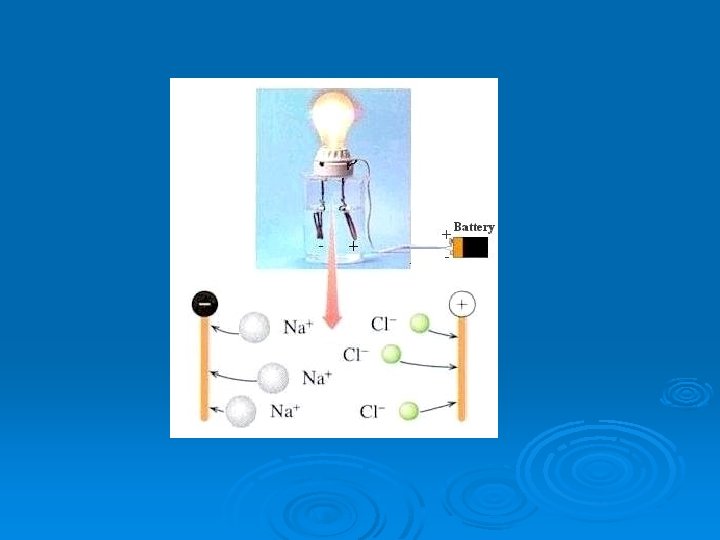
Electrolyte Ø “dissociation” of ionic compounds (salts) into ions to conduct electricity when dissolved in water

Go to the web: Ø http: //www. chem. iastate. edu/group/Green bowe/sections/projectfolder/flashfiles/ther mochem/solution. Salt. html Ø http: //www. northland. cc. mn. us/biology/Biol ogy 1111/animations/dissolve. swf

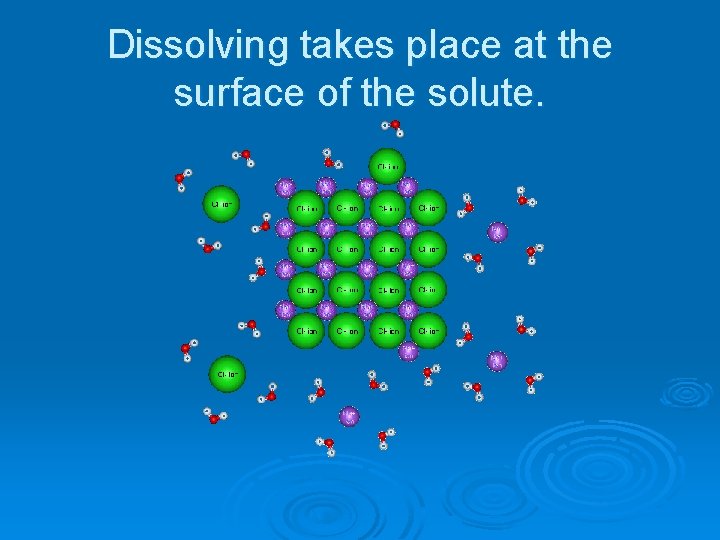
Dissolving takes place at the surface of the solute.




Non-electrolyte Ø Substance does NOT conduct electricity when dissolved in water l Includes all covalent compounds Ø Ex. sugar water

Ø Concentrated vs. dilute Ø Lots of material dissolved vs. very little dissolved

Ø Saturated – solution contains maximum amount of solute Ø Unsaturated – less than maximum Ø Supersaturated – more than maximum l Must be heated

Rock Candy

Alloy Ø Two or more metals dissolved in each other Ø brass = Cu + Zn Ø sterling silver = Ag + Cu

Colligative Properties Ø Dependent on the presence of dissolved particles and their concentration 1) Boiling point elevation – solute particles increase boiling point 2) Freezing point depression – solute particles decrease freezing point

Go to the Web: Ø Boiling point elevation: Ø http: //www. chem. purdue. edu/gchelp/soluti ons/eboil 2. html Ø Freezing point depression: Ø http: //antoine. frostburg. edu/chem/senese/ 101/solutions/faq/why-salt-melts-ice. shtml

Fixing Spaghetti Ø Example of boiling point elevation. Ø Adding salt requires the temperature to be greater than 100 o. C for the water to boil.

Salt on Roads Ø Example of freezing point depression. Ø The temperature must be lower than 32 o. F for the water on the road to freeze.

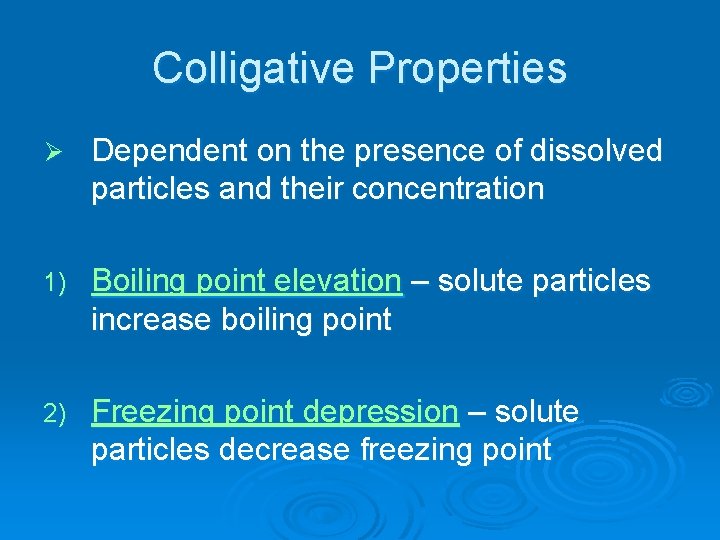
Molarity (M)

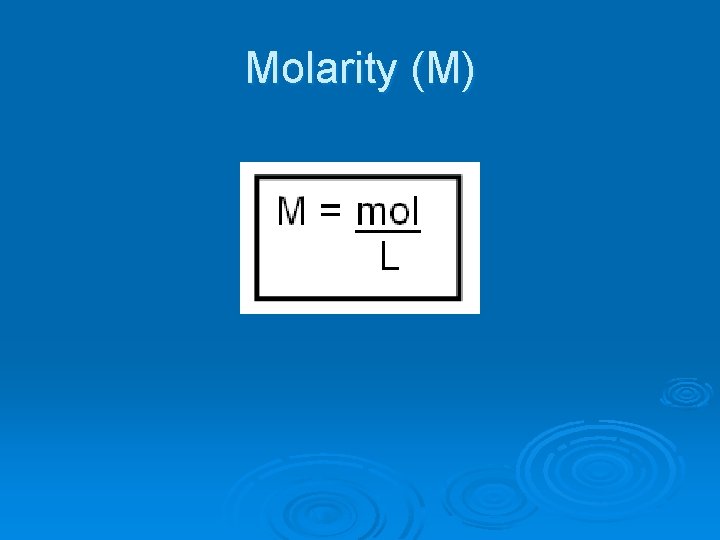
Dilution

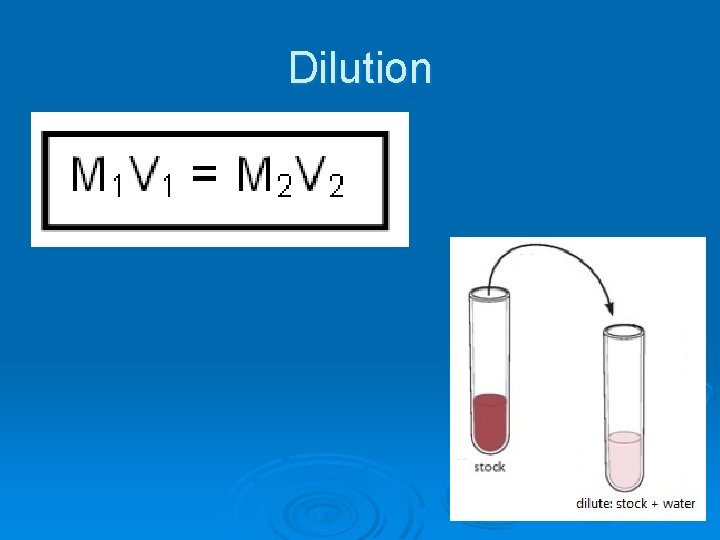
How to dilute a solution

Vocabulary Word: Ø “stock solution” – a solution of a reagent at a standard concentration l Usually the highest concentration

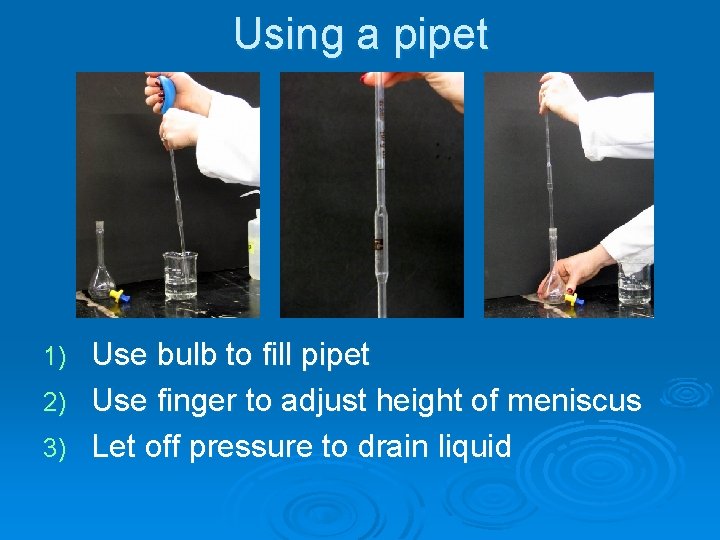
Using a pipet

Using a pipet Use bulb to fill pipet 2) Use finger to adjust height of meniscus 3) Let off pressure to drain liquid 1)