Ch 8 Solutions Acids Bases I How Solutions

Ch. 8 Solutions, Acids, & Bases I. How Solutions Form u u Definitions Types of Solutions Dissolving Rate of Dissolving
A. Definitions u Solution – a mixture that has the same composition throughout the mixture; a homogeneous mixture. Solute - substance being dissolved (in lesser quantity) Solvent – what the solute is dissolved in (in greater quantity)

A. Definitions Solute - KMn. O 4 Solvent - H 2 O

Solutions u Solution – a mixture that has the same composition throughout the mix. u Remember the difference between a mixture and a compound. • Compounds have a fixed composition throughout. • Mixtures can have a variable composition throughout.

A. Definitions u Solubility – The maximum amount of solute that can be dissolved in the solvent at a given temperature.

B. Types of Solutions u. Saturated solutions – maximum amount of solute at a given temperature. u. Unsaturated solutions – less than the maximum amount of solute at a given temperature.
u. Supersaturated solutions – more than the maximum amount of solute at a given temperature; unstable.
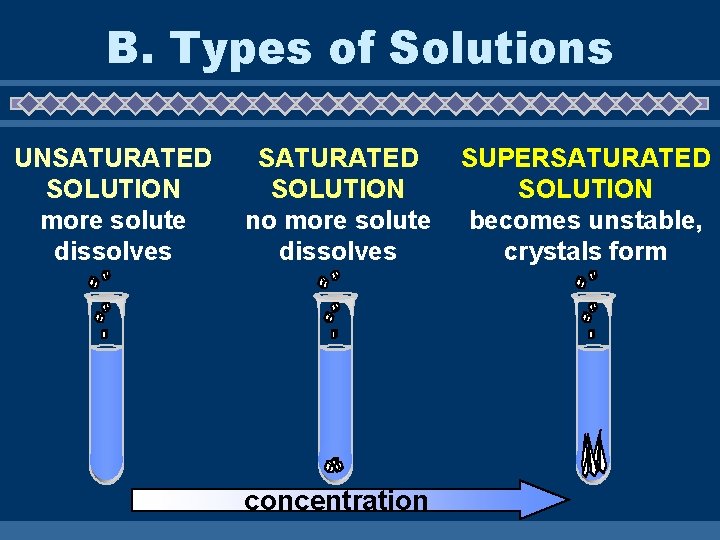
B. Types of Solutions UNSATURATED SOLUTION more solute dissolves SATURATED SOLUTION no more solute dissolves concentration SUPERSATURATED SOLUTION becomes unstable, crystals form

C. Dissolving u Solvation • occurs at the surface of the solute • solvent particles surround solute particles (+/- attraction) • solute particles are pulled into solution

D. Rate of Dissolving u Solids dissolve faster. . . • more stirring • small particle size (increased surface area) • high temperature
Rate of Dissolving To increase rate of dissolving of SOLIDS: u. Heat it u. Crush u. Stir it it

D. Rate of Dissolving u Gases dissolve faster. . . • no shaking or stirring • high pressure • low temperature
u. To make a gas dissolve more quickly in a liquid: • Cool it • Increase the pressure of the gas

Ch. 8 Solutions, Acids, & Bases II. Concentration & Solubility
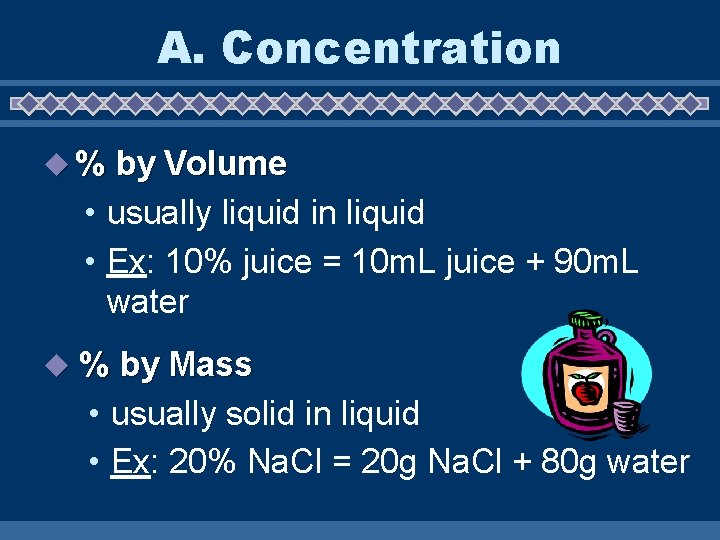
A. Concentration u % by Volume • usually liquid in liquid • Ex: 10% juice = 10 m. L juice + 90 m. L water u % by Mass • usually solid in liquid • Ex: 20% Na. Cl = 20 g Na. Cl + 80 g water

A. Concentration u Concentrated solution • large amount of solute u Dilute solution • small amount of solute

B. Solubility u Solubility • maximum grams of solute that will dissolve in 100 g of solvent at a given temperature • varies with temperature • based on a saturated solution
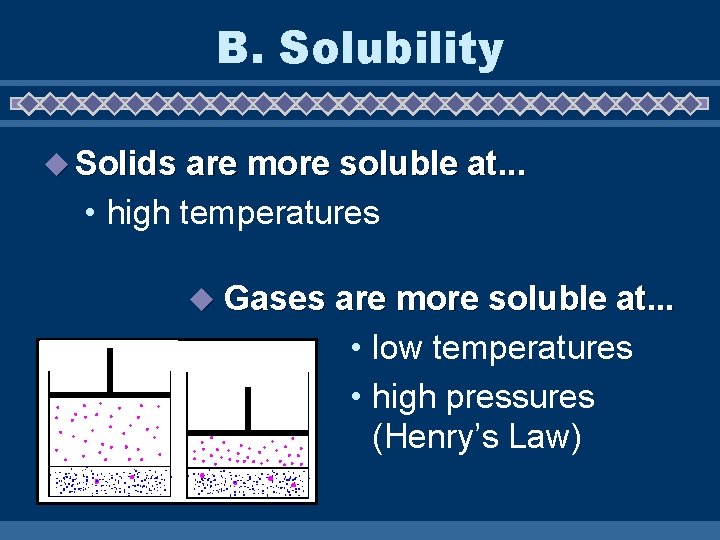
B. Solubility u Solids are more soluble at. . . • high temperatures u Gases are more soluble at. . . • low temperatures • high pressures (Henry’s Law)
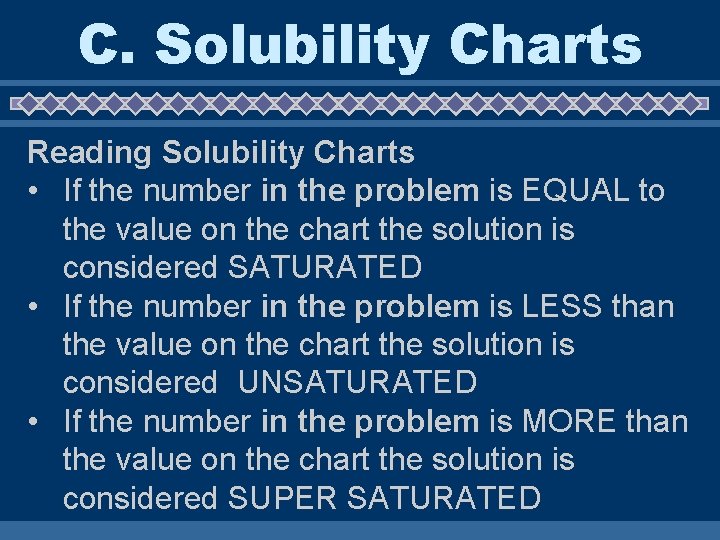
C. Solubility Charts Reading Solubility Charts • If the number in the problem is EQUAL to the value on the chart the solution is considered SATURATED • If the number in the problem is LESS than the value on the chart the solution is considered UNSATURATED • If the number in the problem is MORE than the value on the chart the solution is considered SUPER SATURATED
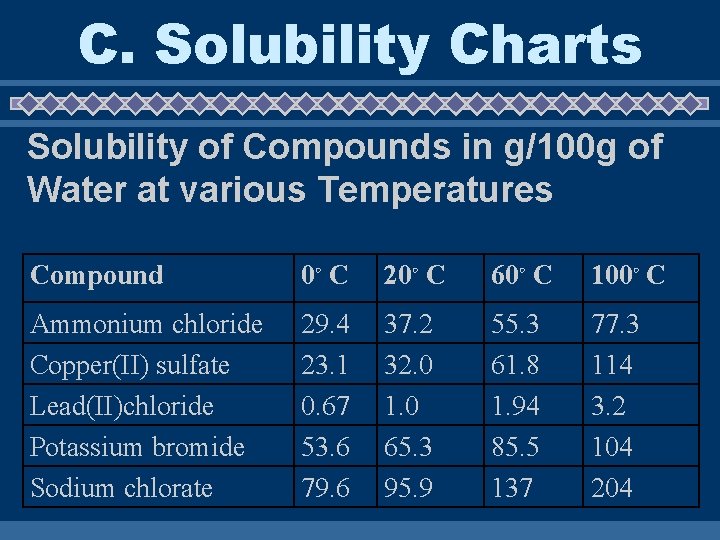
C. Solubility Charts Solubility of Compounds in g/100 g of Water at various Temperatures Compound 0◦ C 20◦ C 60◦ C 100◦ C Ammonium chloride Copper(II) sulfate Lead(II)chloride Potassium bromide Sodium chlorate 29. 4 23. 1 0. 67 53. 6 79. 6 37. 2 32. 0 1. 0 65. 3 95. 9 55. 3 61. 8 1. 94 85. 5 137 77. 3 114 3. 2 104 204

Answer Questions on your paper u Chart 1) How would you classify a solution of 65. 3 g of Saturated potassium bromide at 20ºC? _____ 2) How would you classify a solution of 65. 3 g of Unsaturated potassium bromide at 60ºC? ______ 3) How would you classify a solution of 65. 3 g of Supersaturated potassium bromide at 0ºC? _______ 4) How would you classify a solution of 37 g of Unsaturated ammonium chloride at 20ºC? ______ 5) How would you classify a solution of 2. 5 g of Supersaturated lead (II) chloride at 20ºC? _______
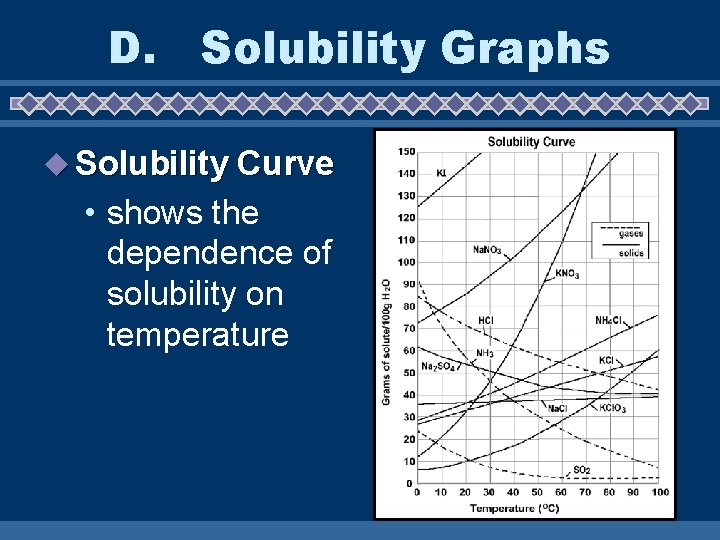
D. Solubility Graphs u Solubility Curve • shows the dependence of solubility on temperature

u Graph 6) How would you classify a solution of 80 g Supersaturated of HCl at 20ºC? ______ 7) How would you classify a solution of 30 g of KNO 3 at 20ºC? Unsaturated _____ 8) How would you classify a solution of 39 g Saturated of Na. Cl at 100ºC? ____ 9) How would you classify a solution of 80 g Unsaturated of Na. NO 3 at 30ºC? _____ 10)How would you classify a solution of 40 g of KCl. O 3 at 80ºC? _____ Unsaturated

11)How many grams of solute would you need to form a saturated solution of NH 4 Cl 50 g at 50ºC? _____ 12)How would you classify a solution of 20 g of Unsaturated SO 2 at 0ºC? ______ 13)How much KI would you need to form a 135 g saturated solution at 10ºC? _____ 14)Which solid decreases in solubility as the temperature increases? Na _______ 2 SO 4
Acid, Bases & Salt Video u 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Pre Test True False True False u 1. 2. 3. 4. 5. 6. 7. 8. 9. Post Test u Post Test Neutralization 10. bitter, slippery, high p. H Base 11. Acids donate Anion hydrogen, Bases Electrolytes accept Hydrogen False 12. An easy way to False gauge is something True is a strong/weak False acid or base False
Ch. 8 Solutions, Acids, & Bases III. Particles in Solution “Like Dissolves Like” u Electrolytes u

A. “Like Dissolves Like” u Polar substances will only dissolve in polar liquids • Rubbing alcohol and water u Nonpolar substances will only dissolve in nonpolar liquids • Oil and butter u Substances that aren’t the same don’t mix. • Oil and water
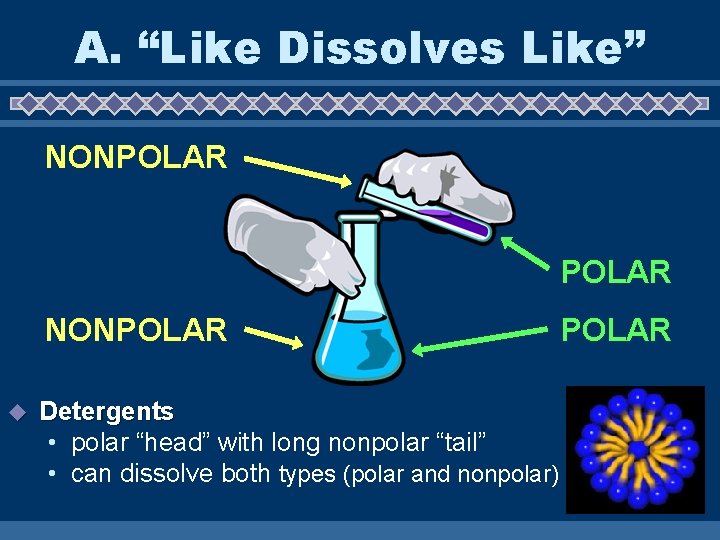
A. “Like Dissolves Like” NONPOLAR u Detergents • polar “head” with long nonpolar “tail” • can dissolve both types (polar and nonpolar) POLAR

B. Electrolyte Ø An electrolyte is a substance that when dissolved in water form ions Ø Electrolytes get their name from the fact that the conduct electricity in water. Ø Example: salt dissolved in water Ø Many sports drinks contain “electrolytes” which are salts dissolved in water
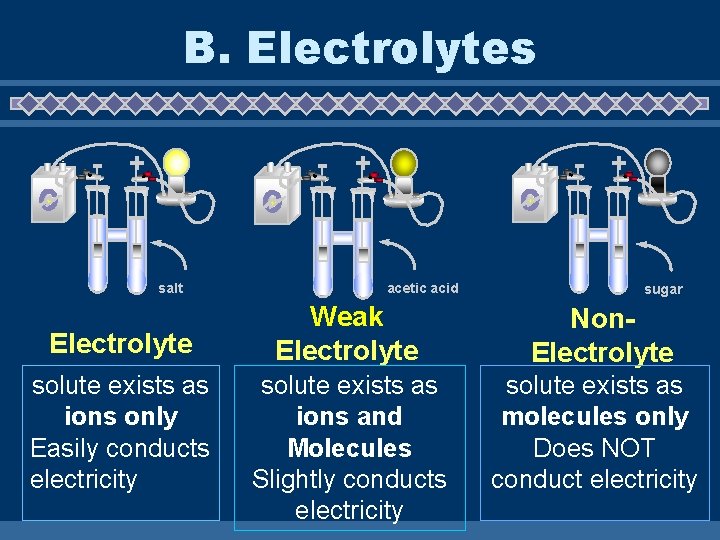
B. Electrolytes - + - salt Electrolyte solute exists as ions only Easily conducts electricity - + + acetic acid sugar Weak Electrolyte Non. Electrolyte solute exists as ions and Molecules Slightly conducts electricity solute exists as molecules only Does NOT conduct electricity
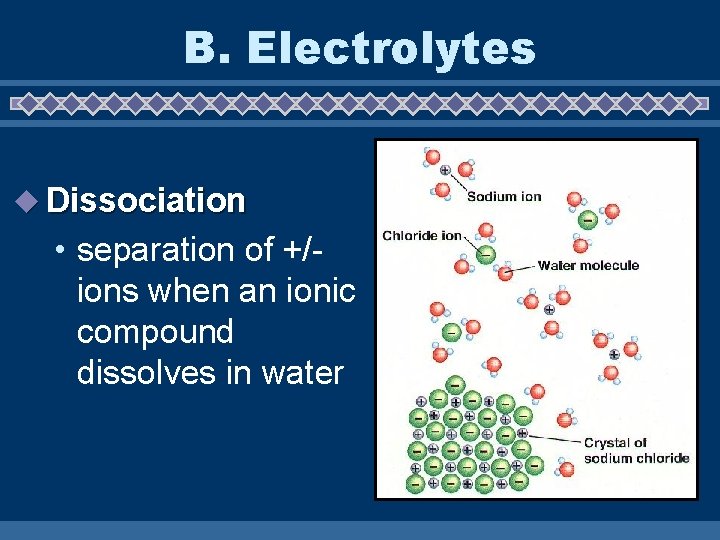
B. Electrolytes u Dissociation • separation of +/ions when an ionic compound dissolves in water
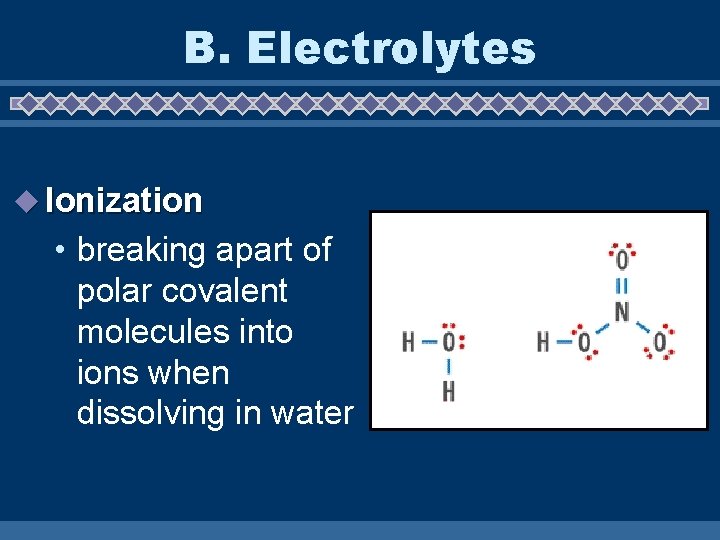
B. Electrolytes u Ionization • breaking apart of polar covalent molecules into ions when dissolving in water

Ch. 8 Solutions, Acids, & Bases IV. Intro to Acids & Bases u u u Definitions Properties Uses
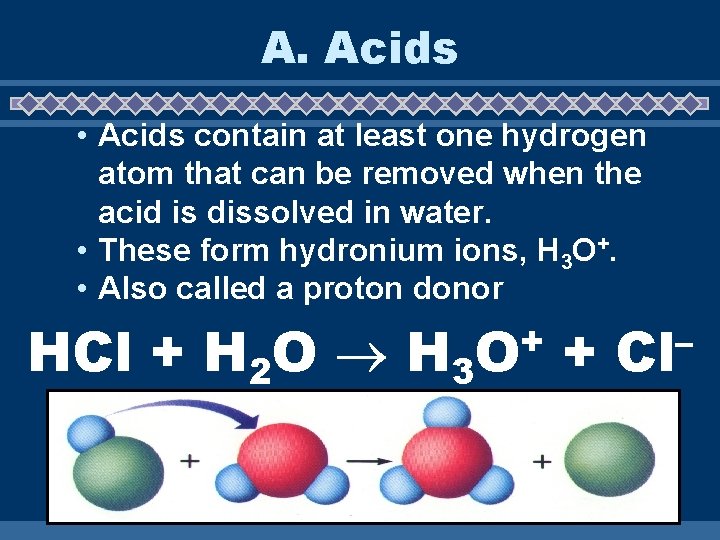
A. Acids • Acids contain at least one hydrogen atom that can be removed when the acid is dissolved in water. • These form hydronium ions, H 3 O+. • Also called a proton donor HCl + H 2 O H 3 + O + – Cl
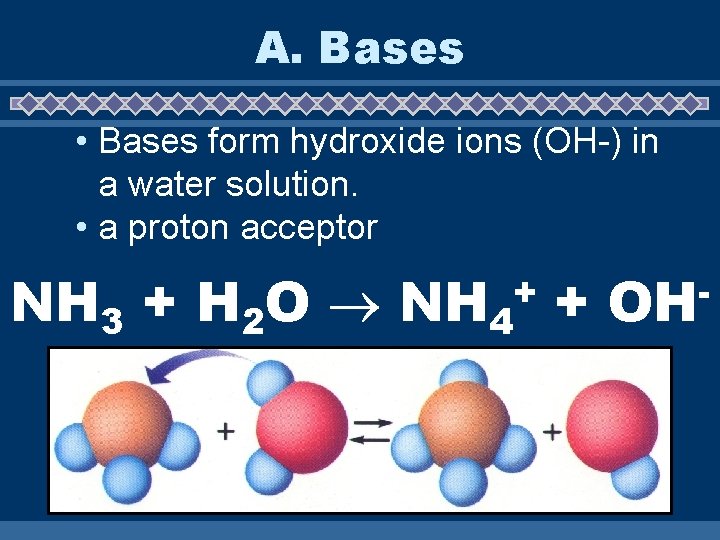
A. Bases • Bases form hydroxide ions (OH-) in a water solution. • a proton acceptor NH 3 + H 2 O NH 4 + + OH
A. Indicators • Indicators are an organic substance that changes color in an acid or base. u Examples: • • litmus - red/blue phenolphthalein - colorless/pink goldenrod - yellow/red cabbage juice - pink/green

B. Properties u sour u p. H taste less than 7 u bitter u p. H taste greater than 7 u corrosive u electrolytes u turn litmus red litmus blue with metals to u slippery feel form H gas u react

C. Uses u H 3 PO 4 –phosphoric acid - soft drinks, fertilizer, detergents u H 2 SO 4 – sulfuric acid - fertilizer, car batteries u HCl – hydrochloric acid - gastric juice u HC 2 H 3 O 2 – acetic acid - vinegar

C. Uses u Na. OH – sodium hydroxide -lye, drain and oven cleaner u Mg(OH)2 – magnesium hydroxide laxative, antacid u NH 3 –ammonia - cleaners, fertilizer
Ch. 8 Solutions, Acids, & Bases V. Strength of Acids & Bases Strength vs. Concentration u. Strong vs. Weak u p. H u

A. Strength vs. Concentration u Strong and weak – tells how easy the acid or base dissociates in solution. u Concentration – The amount of acid or base in a solution. u It is possible to have a dilute concentration of a strong acid that would be less harmful than a concentrated weak acid.

A. Strength of Acids & Bases u The strength of an acid or base depends on how completely a compound separates into ions when dissolved in water. u Ions can carry an electric charge so a strong acid will carry more electricity than weak acid.
B. Strong vs. Weak u Strong Acid/Base - + • 100% ions in water • strong electrolyte • HCl, HNO 3, Na. OH, Li. OH u Weak Acid/Base • few ions in water • weak electrolyte • HC 2 H 3 O 2, NH 3
B. Strong Acids u Acids that ionize almost completely in a solution are strong acids. u Ex: HCl, HNO 3, and H 2 SO 4 u They have a very low p. H (0 -1).

B. Strong Bases u Bases that dissociate completely in a solution are strong bases. u Ex: Na. OH u They have a very high p. H (13 -14)
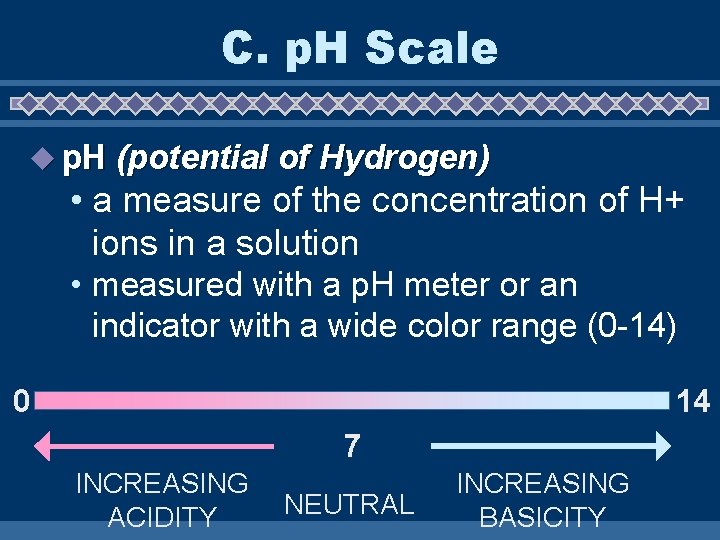
C. p. H Scale u p. H (potential of Hydrogen) • a measure of the concentration of H+ ions in a solution • measured with a p. H meter or an indicator with a wide color range (0 -14) 14 0 7 INCREASING ACIDITY NEUTRAL INCREASING BASICITY
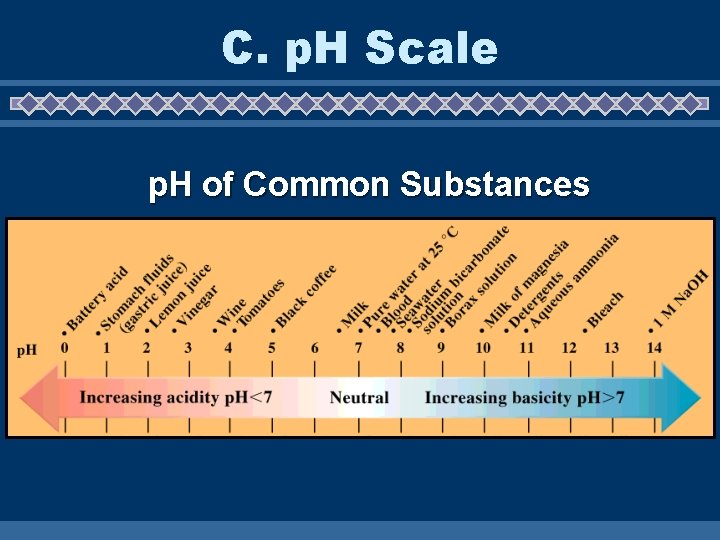
C. p. H Scale p. H of Common Substances
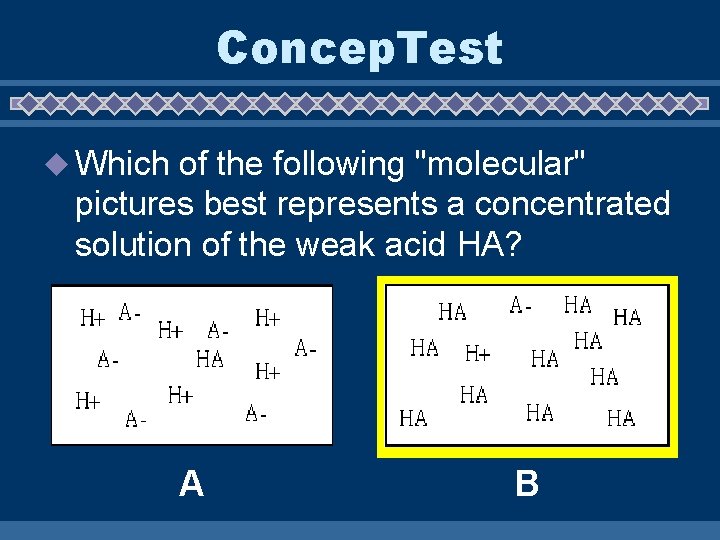
Concep. Test u Which of the following "molecular" pictures best represents a concentrated solution of the weak acid HA? A B

Concep. Test u Is the following statement TRUE or FALSE? • A strong acid has a lower p. H than a weak acid. • True • But: Strong/weak refers to amount of ionization whereas p. H refers to concentration of H+.
Ch. 8 Solutions, Acids, & Bases VI. Neutralization u Neutralization Reaction
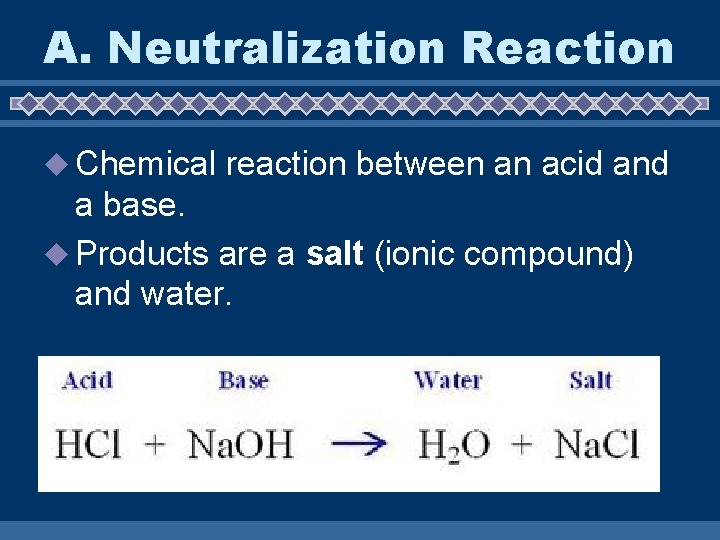
A. Neutralization Reaction u Chemical reaction between an acid and a base. u Products are a salt (ionic compound) and water.

A. Neutralization Reaction ACID + BASE SALT + WATER HCl + Na. OH Na. Cl + H 2 O = Neutralization does not always mean p. H = 7.
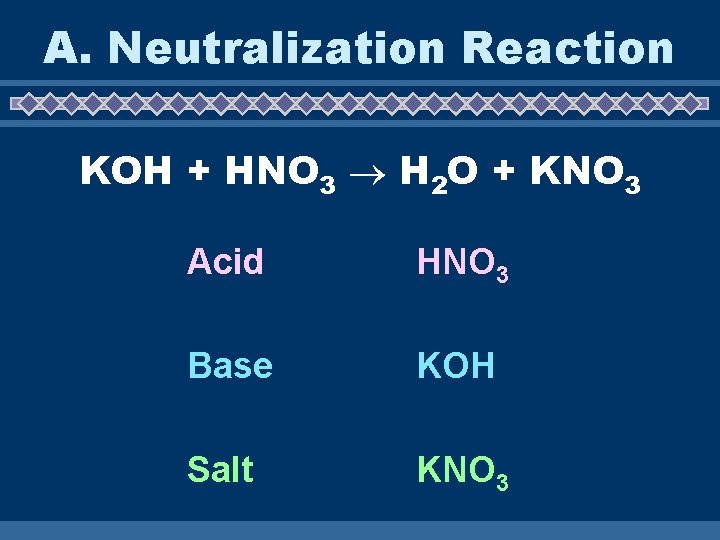
A. Neutralization Reaction KOH + HNO 3 H 2 O + KNO 3 Acid HNO 3 Base KOH Salt KNO 3
- Slides: 53