Solutions Acids and Bases Chapter 8 SMARTStarter Even

Solutions, Acids, and Bases Chapter 8

SMARTStarter Even if you have not studied acids and bases before, you may already know something about them. To tap into this knowledge, identify whether the following are acids or bases. (Hint: Acids and bases have chemical properties that are very different. ) 1. 2. 3. 4. 5. Vinegar Baking soda Soap Orange juice Antacid tablets *Given your categorization of these substances, name one property of acids. *Given your categorization of these substances, name one property of bases.

SMARTStarter What is the difference between a suspension, a solution, and a colloid? Which is which?
SMARTStarter – just a logic test.
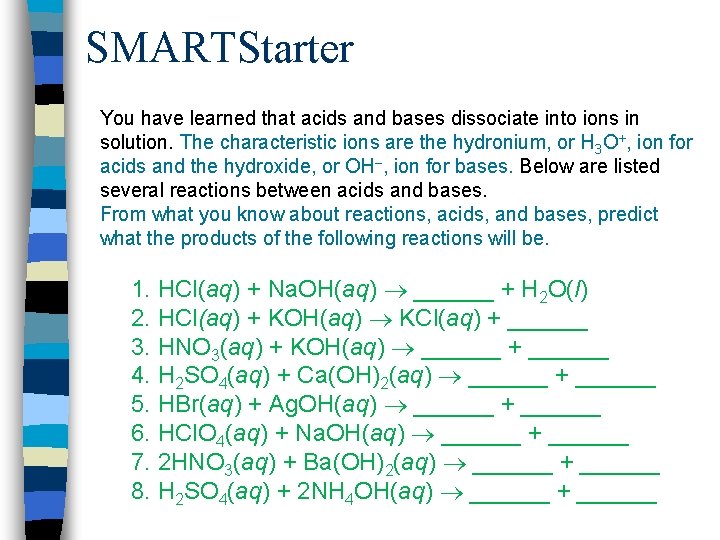
SMARTStarter You have learned that acids and bases dissociate into ions in solution. The characteristic ions are the hydronium, or H 3 O+, ion for acids and the hydroxide, or OH–, ion for bases. Below are listed several reactions between acids and bases. From what you know about reactions, acids, and bases, predict what the products of the following reactions will be. 1. HCl(aq) + Na. OH(aq) ______ + H 2 O(l) 2. HCl(aq) + KOH(aq) KCl(aq) + ______ 3. HNO 3(aq) + KOH(aq) ______ + ______ 4. H 2 SO 4(aq) + Ca(OH)2(aq) ______ + ______ 5. HBr(aq) + Ag. OH(aq) ______ + ______ 6. HCl. O 4(aq) + Na. OH(aq) ______ + ______ 7. 2 HNO 3(aq) + Ba(OH)2(aq) ______ + ______ 8. H 2 SO 4(aq) + 2 NH 4 OH(aq) ______ + ______
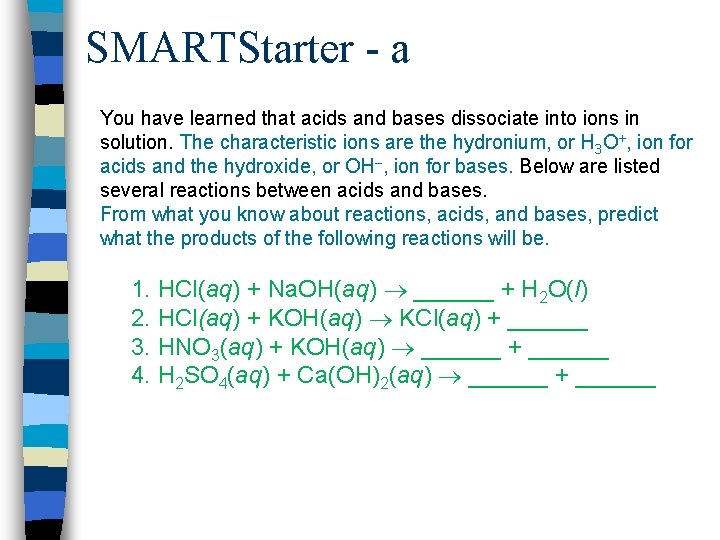
SMARTStarter - a You have learned that acids and bases dissociate into ions in solution. The characteristic ions are the hydronium, or H 3 O+, ion for acids and the hydroxide, or OH–, ion for bases. Below are listed several reactions between acids and bases. From what you know about reactions, acids, and bases, predict what the products of the following reactions will be. 1. HCl(aq) + Na. OH(aq) ______ + H 2 O(l) 2. HCl(aq) + KOH(aq) KCl(aq) + ______ 3. HNO 3(aq) + KOH(aq) ______ + ______ 4. H 2 SO 4(aq) + Ca(OH)2(aq) ______ + ______
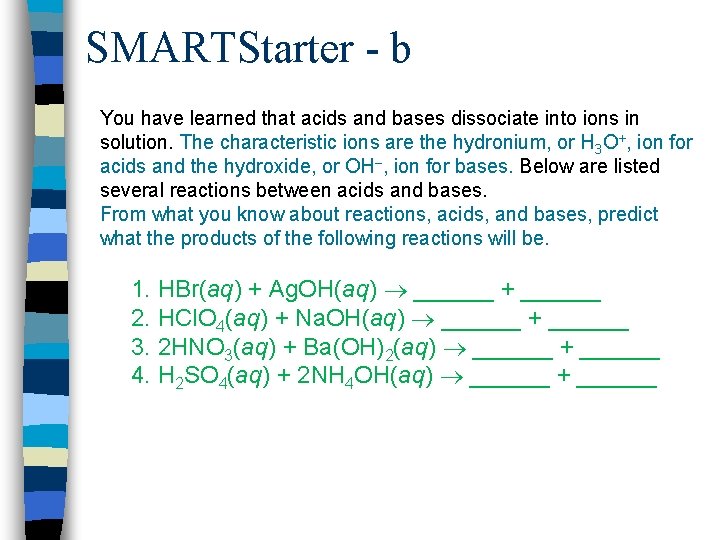
SMARTStarter - b You have learned that acids and bases dissociate into ions in solution. The characteristic ions are the hydronium, or H 3 O+, ion for acids and the hydroxide, or OH–, ion for bases. Below are listed several reactions between acids and bases. From what you know about reactions, acids, and bases, predict what the products of the following reactions will be. 1. HBr(aq) + Ag. OH(aq) ______ + ______ 2. HCl. O 4(aq) + Na. OH(aq) ______ + ______ 3. 2 HNO 3(aq) + Ba(OH)2(aq) ______ + ______ 4. H 2 SO 4(aq) + 2 NH 4 OH(aq) ______ + ______

SMARTStarter - a Many acids, bases, and salts are used daily in our homes. Based on what you know about the properties of acids, bases, and salts, answer the following items. 1. Some green apples are particularly sour. Is this likely to be due to an excess of acid or base? 2. Baking soda reacts with vinegar. Is baking soda an acid or a base? 3. What products besides carbon dioxide remain after vinegar and baking soda react completely?

SMARTStarter - b Many acids, bases, and salts are used daily in our homes. Based on what you know about the properties of acids, bases, and salts, answer the following items. 1. Given that one approach to an upset stomach is to take an antacid, are the chemicals secreted into the stomach for digestion likely to be acids or bases? 2. Drain cleaners that contain lye react with fatty acids in clogs. Given that, are drain cleaners that contain lye acids or bases? 3. Why does the clog dissolve after the reaction of the fatty acids with lye? 4. Many common window cleaners contain ammonia, a base. Given that, is most dirt on window slightly acidic or slightly basic?

Part of the solution? Solute v The substance that is dissolved into the solution. v examples: n • Sugar in kool-aid • Salt in salt water • CO 2 in pop Solvent v The substance that does the dissolving in a solution. v examples: n • Most common is water.

Dissolving = creating a solution Ø Three ways to dissolve a solute in a solvent: 1. Dissociation 2. Dispersion 3. Ionization
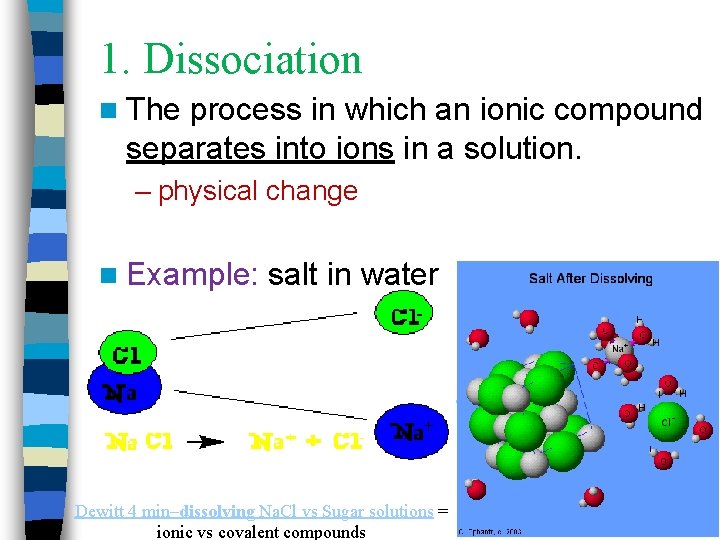
1. Dissociation n The process in which an ionic compound separates into ions in a solution. – physical change n Example: salt in water 1 min. animation Dewitt 4 min–dissolving Na. Cl vs Sugar solutions = ionic vs covalent compounds
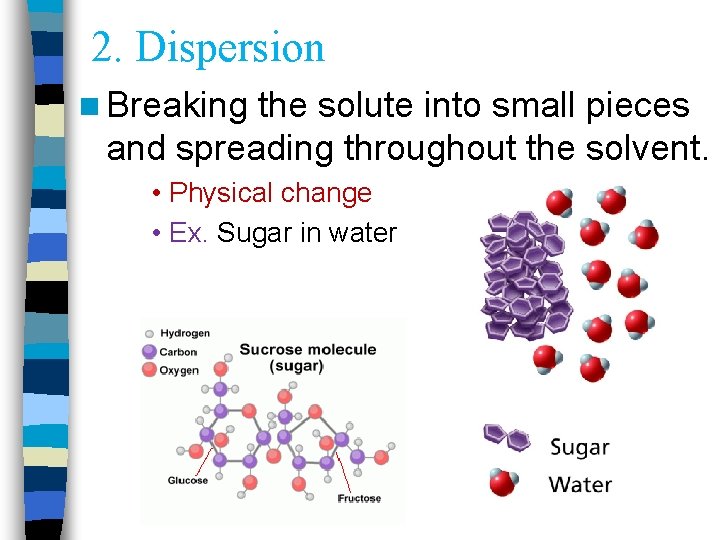
2. Dispersion n Breaking the solute into small pieces and spreading throughout the solvent. • Physical change • Ex. Sugar in water
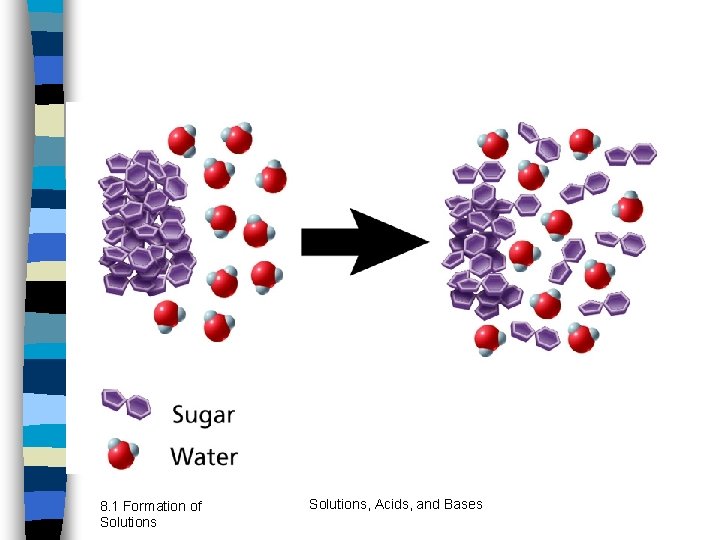
8. 1 Formation of Solutions, Acids, and Bases
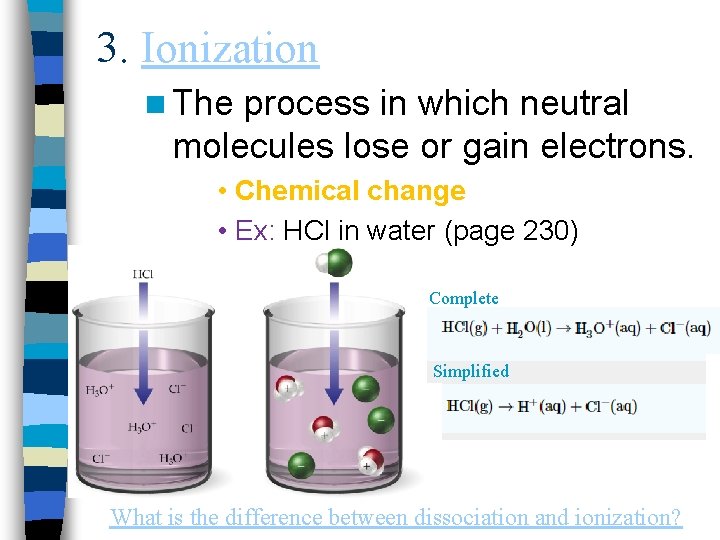
3. Ionization n The process in which neutral molecules lose or gain electrons. • Chemical change • Ex: HCl in water (page 230) Complete Simplified What is the difference between dissociation and ionization?
Properties of Liquid Solutions n Three physical properties of a solution that can differ from those of its solute and solvent are: » conductivity » freezing point » boiling point 8. 1 Formation of Solutions, Acids, and Bases
Conductivity n Many solutions can conduct an electric current if electrolytes are present. (ions) – Electrolytes = substances that will conduct an electric current when dissolved. – Ex. Na. Cl, KCl, Mg. Br 2
Freezing Point Depression n Lowering the freezing point of water by the addition of a solute – ex. salt. • Used on icy roads in winter • Ice-cream
Boiling point elevation The addition of a solute to a liquid solvent will usually raise the boiling point of the solvent. n Adding salt to boil water when cooking n
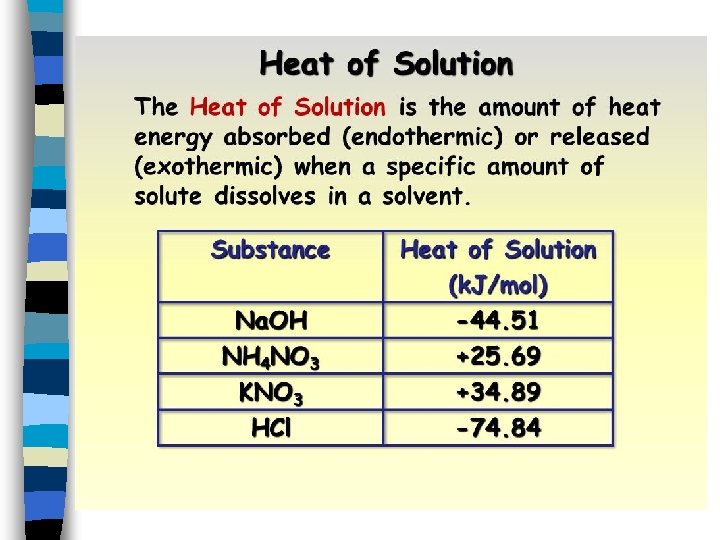
Heat of Solution n A measure of the amount of energy either absorbed or released when a solute dissolves in a solvent. n Can be endothermic or exothermic.

Potential Lab: Alka-Seltzer Solution Lab What factors will speed up the dissolving process? i. e. How does temperature affect dissolving time? How does volume of solvent affect dissolving time? How does type of solvent affect dissolving time? How does stirring affect dissolving time? How does pressure affect dissolving time? How does salt concentration affect dissolving time? How does sugar concentration affect dissolving time? How does ? ? affect the dissolving time?
Rate of Solution n Speed at which solute dissolves in a solvent. Speed is affected by three factors…
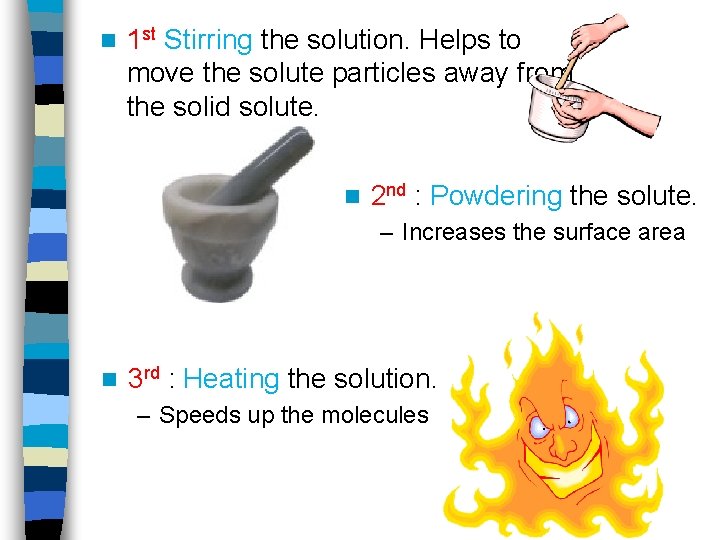
n 1 st Stirring the solution. Helps to move the solute particles away from the solid solute. n 2 nd : Powdering the solute. – Increases the surface area n 3 rd : Heating the solution. – Speeds up the molecules
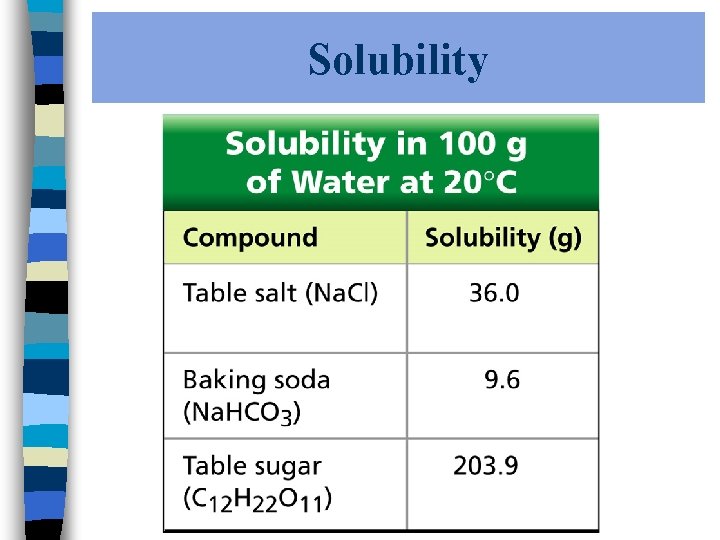
Solubility
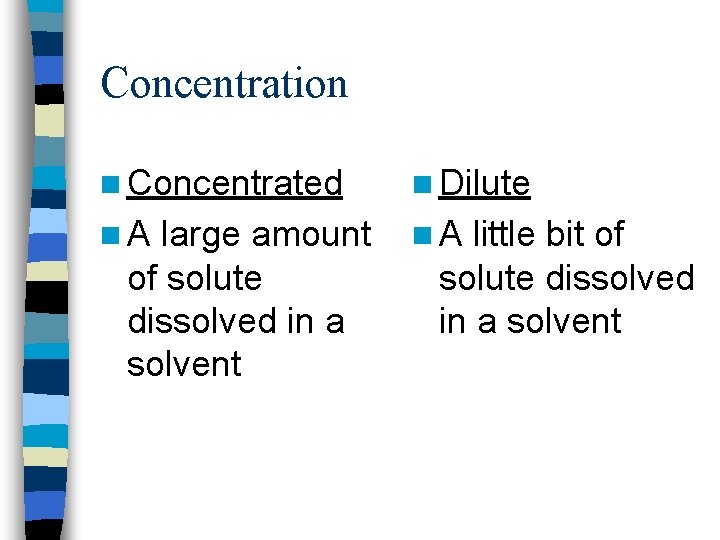
Concentration n Concentrated n Dilute n A large amount n A little bit of solute dissolved in a solvent

Saturation n A solution that contains all the solute it can possibly hold at a given temperature is said to be saturated. n Unsaturated = contains less solute than it can possibly hold n Supersaturated = a solution that holds more solute than it should at a given temperature.

Supersaturation example: n Sodium acetate in water. n Used in commercial hand warmers.
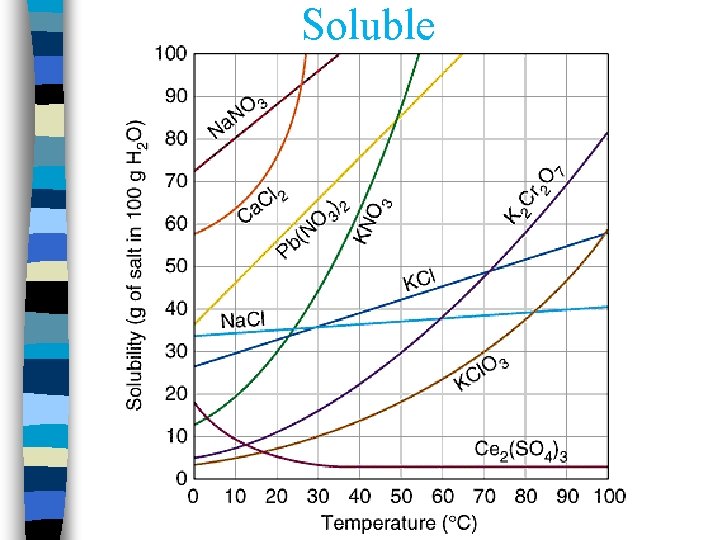
Soluble

Insoluble A substance that will NOT dissolve in water.
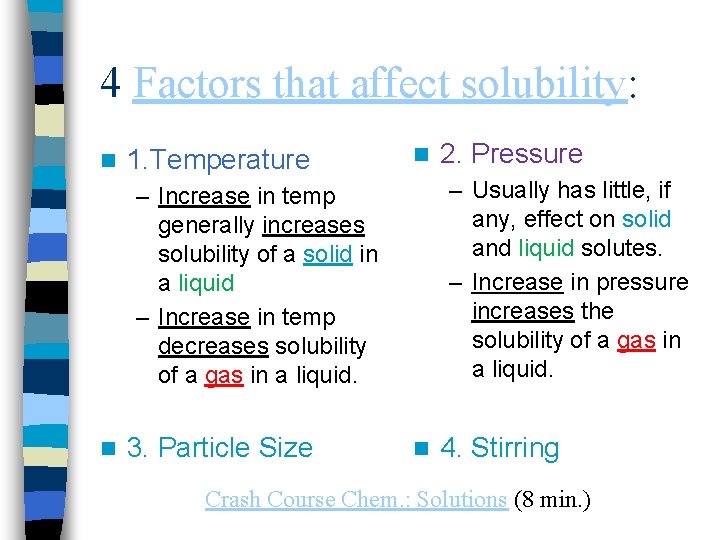
4 Factors that affect solubility: n 1. Temperature n – Usually has little, if any, effect on solid and liquid solutes. – Increase in pressure increases the solubility of a gas in a liquid. – Increase in temp generally increases solubility of a solid in a liquid – Increase in temp decreases solubility of a gas in a liquid. n 3. Particle Size 2. Pressure n 4. Stirring Crash Course Chem. : Solutions (8 min. )

“Like dissolves like” n Nonpolar solvents will dissolve nonpolar solutes. – examples: – benzene (C 6 H…) & acetone – any of the homo-nuclear diatomic elements: H 2, N 2, O 2, Cl 2 (These are truly nonpolar molecules. ) – carbon dioxide - CO. . n Polar solvents will dissolve polar solutes – examples: • Ethanol (drinking alcohol) • Acetic Acid (vinegar has a lot of this) • Ammonium (Common in things like fertilizers)

Specific Concentration n Can be defined as percent by volume or percent by mass • Example: – 3% hydrogen peroxide – 25% fruit juice.
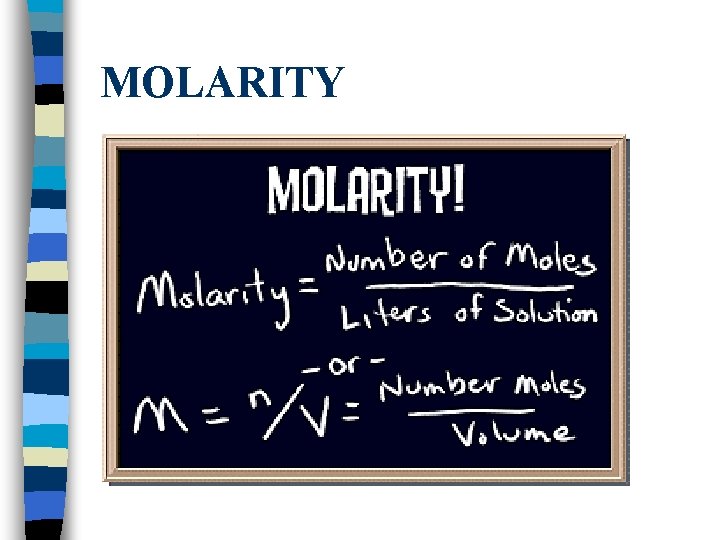
MOLARITY

Practice… Example: Table sugar , C 12 H 22 O 11, has a molar mass of 342 grams. To make a 1 -molar (1 M) solution of table sugar water, add 342 g of sugar to enough water to make one liter of solution. Dewitt Examples – 9 min. Part II – 11 min.
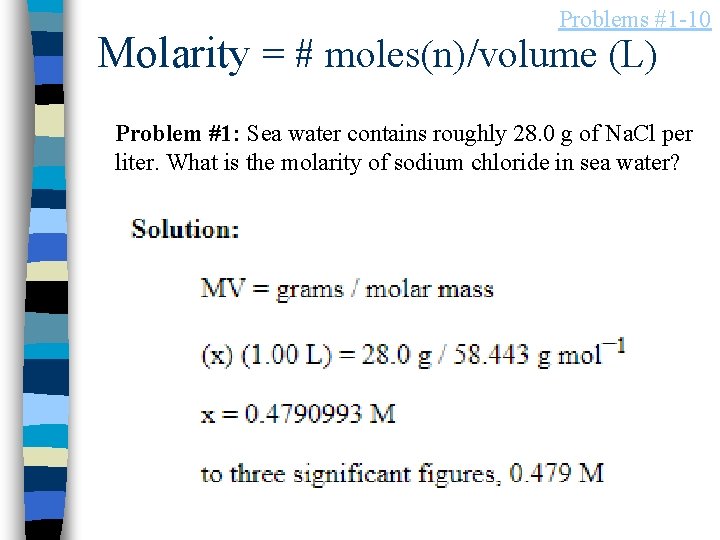
Problems #1 -10 Molarity = # moles(n)/volume (L) Problem #1: Sea water contains roughly 28. 0 g of Na. Cl per liter. What is the molarity of sodium chloride in sea water?
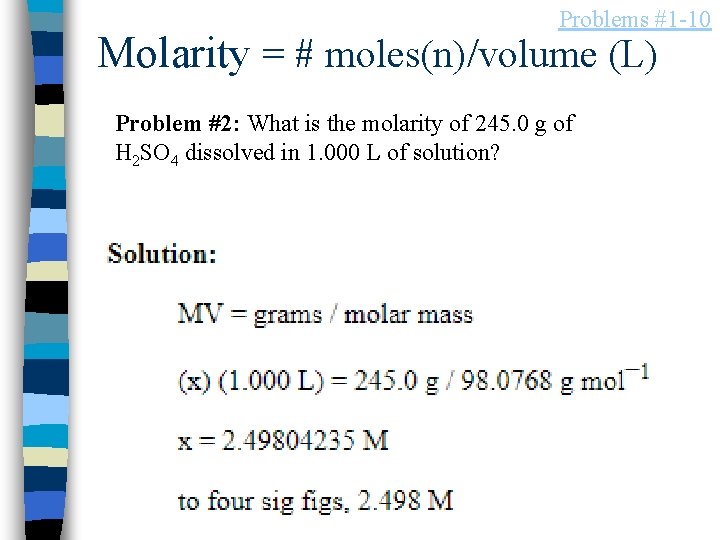
Problems #1 -10 Molarity = # moles(n)/volume (L) Problem #2: What is the molarity of 245. 0 g of H 2 SO 4 dissolved in 1. 000 L of solution?
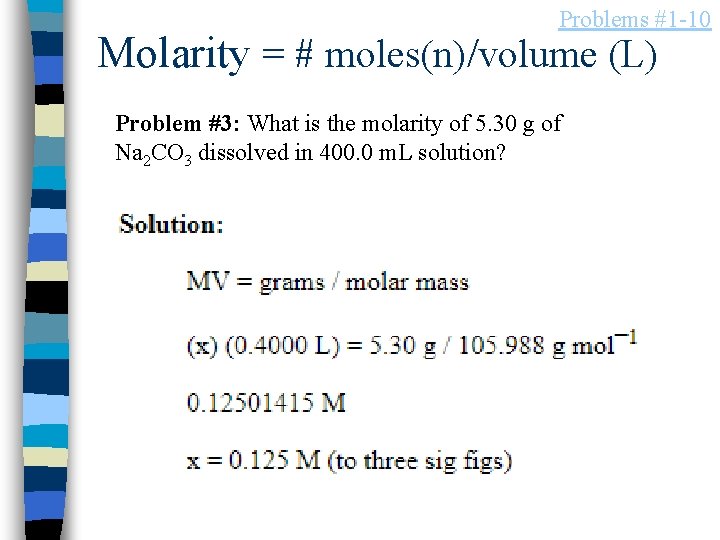
Problems #1 -10 Molarity = # moles(n)/volume (L) Problem #3: What is the molarity of 5. 30 g of Na 2 CO 3 dissolved in 400. 0 m. L solution?
Acids & Bases Introduction: n CARPI Reading 10 Q Quiz
Acids n Very important chemicals in everyday life processes.

Properties of acids: Sour taste (never taste a chemical) 2. All contain hydrogen 3. Also called “proton donors” 4. React with active metals to produce hydrogen gas. (exp. 21) 1. Zn + 2 HCl Zn. Cl 2 + H 2
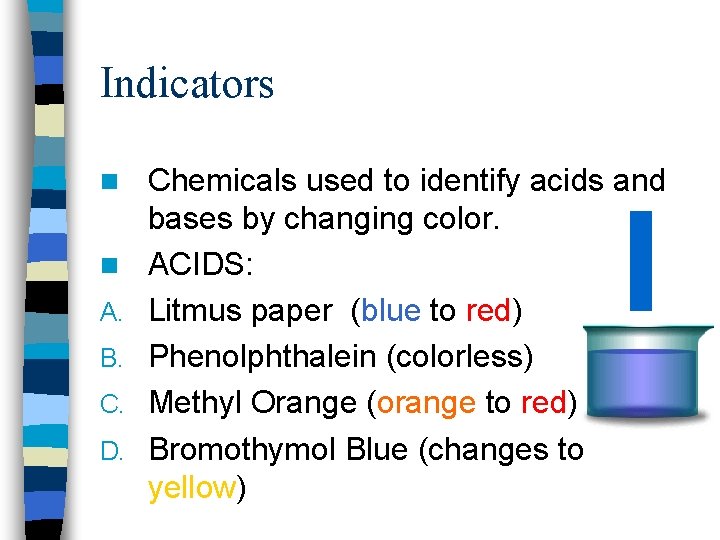
Indicators n n A. B. C. D. Chemicals used to identify acids and bases by changing color. ACIDS: Litmus paper (blue to red) Phenolphthalein (colorless) Methyl Orange (orange to red) Bromothymol Blue (changes to yellow)

8. 3 Properties of Acids and Bases Solutions, Acids, and Bases

Common acids n Sulfuric (H 2 SO 4) used in car batteries n Nitric (HNO 3) also fertilizers n Hydrochloric (HCl) stomach acid n Carbonic (H 2 CO 3) carbonated drinks n Acetic (HC 2 H 3 O 2) vinegar

Bases n Also very important in everyday processes.

Properties of Bases: n Taste bitter; feel slippery n Contain hydroxide (OH) ions. n Known as “proton acceptors” n Phenolphthalein turns bright pink n Red litmus paper turns blue n Bromothymol blue turns blue n Methyl orange turns yellow

Common bases: n Sodium hydroxide Na. OH making soap; drain cleaners n Potassium hydroxide KOH battery electrolyte n Calcium hydroxide Ca(OH)2 making plaster and drywall n Magnesium hydroxide Mg(OH)2 antacids

What happens when an acid and a base combine? n Always forms water and a salt. n Salt = ionic compound formed when a positive ion of a base combines with a negative ion of an acid. n Neutralization. n These are double replacement reactions. Examples: (on board)

Proton Donors and Acceptors
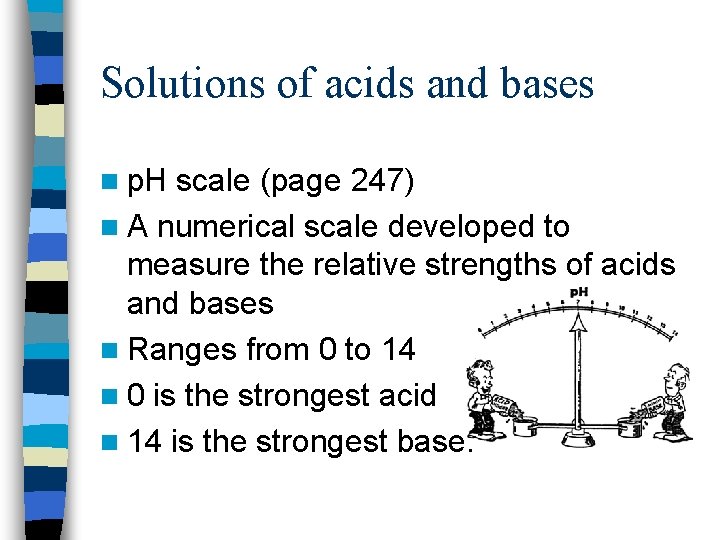
Solutions of acids and bases n p. H scale (page 247) n A numerical scale developed to measure the relative strengths of acids and bases n Ranges from 0 to 14 n 0 is the strongest acid n 14 is the strongest base.
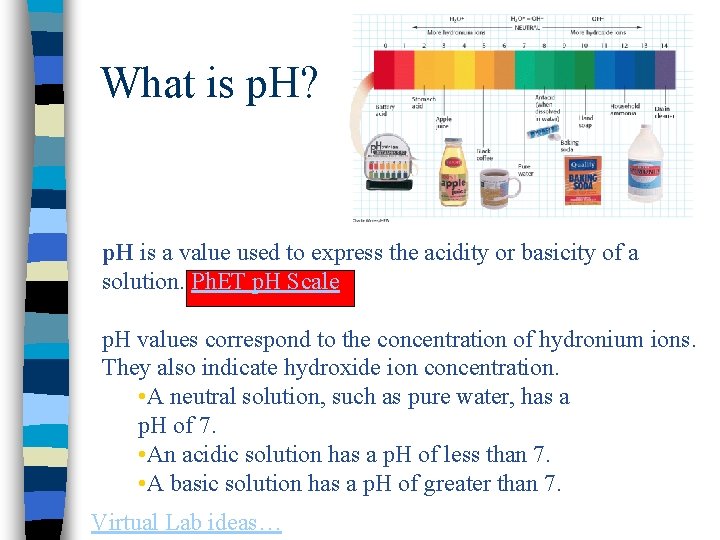
What is p. H? p. H is a value used to express the acidity or basicity of a solution. Ph. ET p. H Scale p. H values correspond to the concentration of hydronium ions. They also indicate hydroxide ion concentration. • A neutral solution, such as pure water, has a p. H of 7. • An acidic solution has a p. H of less than 7. • A basic solution has a p. H of greater than 7. Virtual Lab ideas…

Water… n Water ionizes slightly. – 2 H 2 O H 3 O+ + OH− n The arrow to the left is longer than the arrow to the right showing that water contains more molecules than ions. n Water is neutral because is has equal concentrations of hydronium and hydroxide ions.
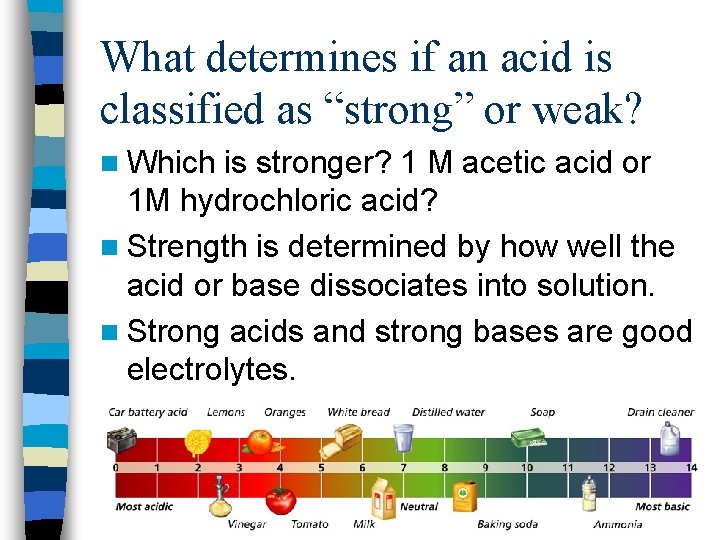
What determines if an acid is classified as “strong” or weak? n Which is stronger? 1 M acetic acid or 1 M hydrochloric acid? n Strength is determined by how well the acid or base dissociates into solution. n Strong acids and strong bases are good electrolytes.
Buffers A mixture of a weak acid or weak base with its salt. n Resists large changes in p. H. n Examples: n – Bicarbonate ions in your blood stream.

Electrolytes n An electrolyte is a substance that ionizes or dissociates when it dissolves in water. n The electrolytes in sports drinks help restore balance of ions in the body.

Real Life: Fuel Cells & Batteries

Self-Tutor Video Helps n Crash Course Chem. : Solutions (8 min. ) n More Depth: Molarity, Solutions, Concentrations & Dilutions (10 min. )

Assignment n Pages 257 -258 n 1 -10, 11, 13, 16, 20, 22, 23, 24, 27, 30, 31, 32

Chapter 8 Section 1 Acids, Bases, and p. H Math Skills Determining p. H Determine the p. H of a 0. 0001 M solution of the strong acid HCl dissolved in water. 1. List the given and unknown values. Given: concentration of HCl in solution = 0. 0001 M Unknown: p. H 2. Determine the molar concentration of hydroxide ions. HCl is completely ionized into H 3 O+ and Cl– ions. concentration of H 3 O+ = concentration of HCl = 0. 0001 M = 1 10– 4 M Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
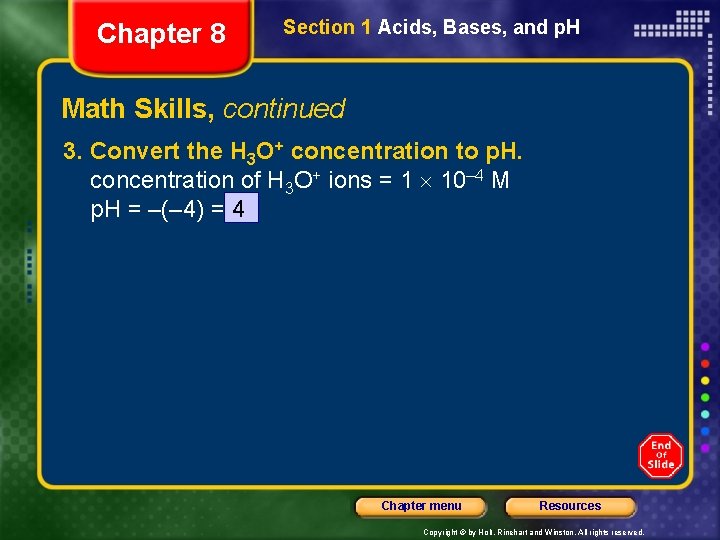
Chapter 8 Section 1 Acids, Bases, and p. H Math Skills, continued 3. Convert the H 3 O+ concentration to p. H. concentration of H 3 O+ ions = 1 10– 4 M p. H = –(– 4) = 4 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 8 Standardized Test Prep Understanding Concepts 1. What is the p. H of a solution with a hydronium ion concentration of 1 10– 13 M? A. B. C. D. – 13 1 7 13 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 8 Standardized Test Prep Understanding Concepts 1. What is the p. H of a solution with a hydronium ion concentration of 1 10– 13 M? A. B. C. D. – 13 1 7 13 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Standardized Test Prep Understanding Concepts 2. Which of the following is true of weak acids? F. They are less soluble in water than strong acids. G. They do not ionize as completely in water as strong acids. H. They have a p. H value that is lower than that of strong acids. I. They do not react with bases to form a salt as do strong acids. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Standardized Test Prep Understanding Concepts 2. Which of the following is true of weak acids? F. They are less soluble in water than strong acids. G. They do not ionize as completely in water as strong acids. H. They have a p. H value that is lower than that of strong acids. I. They do not react with bases to form a salt as do strong acids. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Standardized Test Prep Understanding Concepts 3. Which of the following p. H ranges would include the equivalence point of the titration of a strong acid with a weak base? A. B. C. D. p. H 2 to p. H 6 to p. H 8 exactly p. H 7 exactly p. H 8 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Standardized Test Prep Understanding Concepts 3. Which of the following p. H ranges would include the equivalence point of the titration of a strong acid with a weak base? A. B. C. D. p. H 2 to p. H 6 to p. H 8 exactly p. H 7 exactly p. H 8 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Standardized Test Prep Understanding Concepts 4. What property of detergents makes them more useful than soap for washing clothes and dishes in many parts of the country? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Standardized Test Prep Understanding Concepts 4. What property of detergents makes them more useful than soap for washing clothes and dishes in many parts of the country? Answer: Detergents do not form insoluble salts with calcium as do soaps, so they work better in hard water. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Standardized Test Prep Understanding Concepts 5. How can you determine by tasting which foods have a high concentration of one or more acidic compounds? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Standardized Test Prep Understanding Concepts 5. How can you determine by tasting which foods have a high concentration of one or more acidic compounds? Answer: Acidic compounds have a sour taste. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Standardized Test Prep Reading Skills Nitrogen and sulfur compounds formed by the combustion of fossil fuels react with oxygen and water in the air to form acids. These acids dissolve in water, forming acid precipitation, which can react with objects on which it falls. Limestone statues and decorations on buildings in areas with acidic rainfall have been severely damaged by the acid. On the other hand, granite statues and carvings tend to suffer much less damage under the same conditions. 6. Assess why one type of stone, limestone, is affected by acid precipitation much more than another type of stone, granite. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 8 Standardized Test Prep Reading Skills, continued 6. Assess why one type of stone, limestone, is affected by acid precipitation much more than another type of stone, granite. Answer: Limestone reacts more with acid than granite. Therefore, the compounds that make up limestone must be basic, and those in granite are not. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
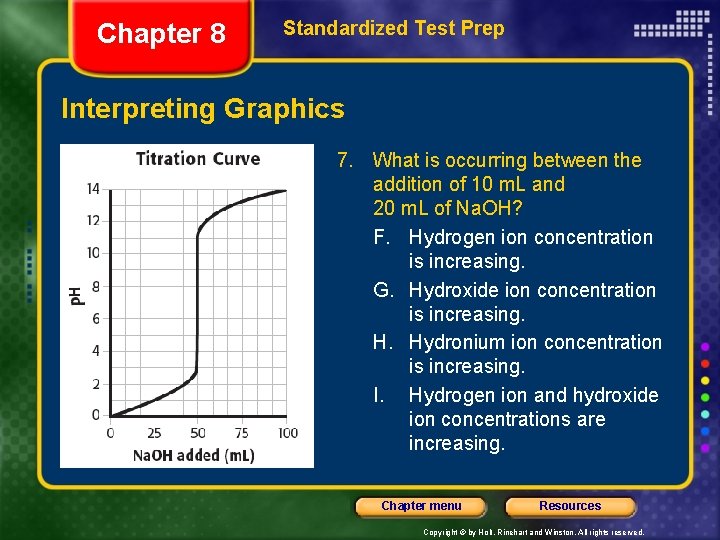
Chapter 8 Standardized Test Prep Interpreting Graphics 7. What is occurring between the addition of 10 m. L and 20 m. L of Na. OH? F. Hydrogen ion concentration is increasing. G. Hydroxide ion concentration is increasing. H. Hydronium ion concentration is increasing. I. Hydrogen ion and hydroxide ion concentrations are increasing. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
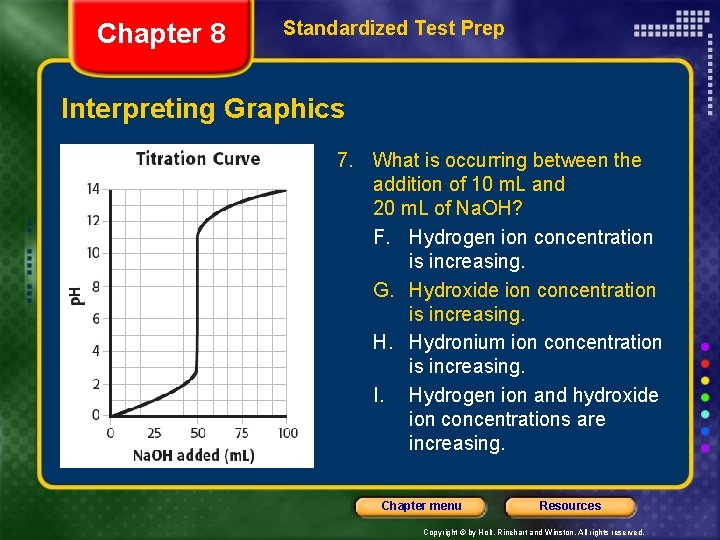
Chapter 8 Standardized Test Prep Interpreting Graphics 7. What is occurring between the addition of 10 m. L and 20 m. L of Na. OH? F. Hydrogen ion concentration is increasing. G. Hydroxide ion concentration is increasing. H. Hydronium ion concentration is increasing. I. Hydrogen ion and hydroxide ion concentrations are increasing. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
- Slides: 74