CHAPTER THREE 12 Physical Properties of Solutions Chapter











- Slides: 46

CHAPTER THREE (12) Physical Properties of Solutions

Chapter 3 / Physical Properties of Solutions Chapter Three outline Chapter Three Contains: 3. 1 Types of Solutions 3. 2 A Molecular View of the Solution Process 3. 3 Concentration Units 3. 4 The Effect of Temperature on Solubility 3. 5 The Effect of Pressure on the Solubility of Gases 3. 6 Colligative Properties of Nonelectrolyte Solutions 3. 7 Colligative Properties of Electrolyte Solutions 3. 8 Colloids 2

Chapter 3 / Physical Properties of Solutions 3. 1 Types of Solutions n n A solution is a homogenous mixture of 2 or more substances The solute is(are) the substance(s) present in the smaller amount(s) The solvent is the substance present in the larger amount Our focus in this chapter will be on solutions involving at least one liquid component—that is, gas-liquid, liquid-liquid, and solid-liquid solutions 3

Chapter 3 / Physical Properties of Solutions 3. 1 Types of Solutions n n n There are 3 types of solutions based on their capacity to dissolve a solute : A saturated solution contains the maximum amount of a solute that will dissolve in a given solvent at a specific temperature. An unsaturated solution contains less solute than the solvent has the capacity to dissolve at a specific temperature. A supersaturated solution contains more solute than is present in a saturated solution at a specific temperature. (not very stable) Sodium acetate crystals rapidly form when a seed crystal is added to a supersaturated solution of sodium acetate. 4

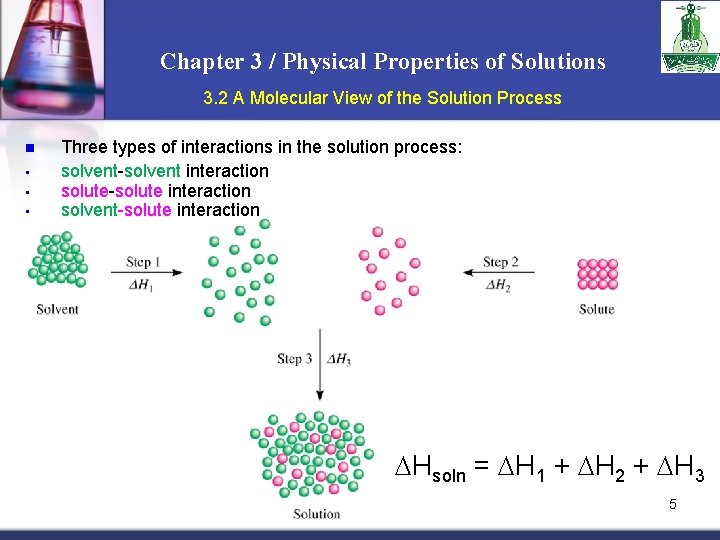
Chapter 3 / Physical Properties of Solutions 3. 2 A Molecular View of the Solution Process n • • • Three types of interactions in the solution process: solvent-solvent interaction solute-solute interaction solvent-solute interaction Hsoln = H 1 + H 2 + H 3 5

Chapter 3 / Physical Properties of Solutions 3. 2 A Molecular View of the Solution Process “like dissolves like” n • Two substances with similar intermolecular forces are likely to be soluble in each other. non-polar molecules are soluble in non-polar solvents (most organic compounds are non-polar molecules) CCl 4 in C 6 H 6 • polar molecules are soluble in polar solvents C 2 H 5 OH in H 2 O • n ionic compounds are more soluble in polar solvents Na. Cl in H 2 O or NH 3 (l) Solvation is the process in which an ion or a molecule is surrounded by solvent molecules arranged in a specific manner. The process is called hydration when 6 the solvent is water.

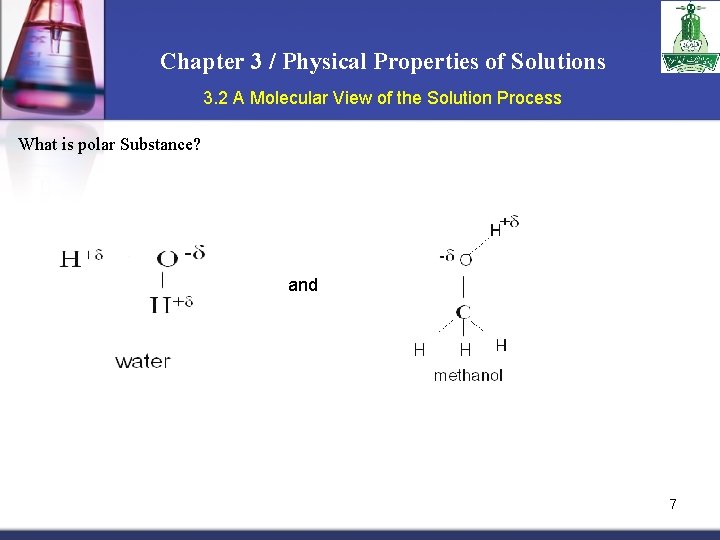
Chapter 3 / Physical Properties of Solutions 3. 2 A Molecular View of the Solution Process What is polar Substance? and 7

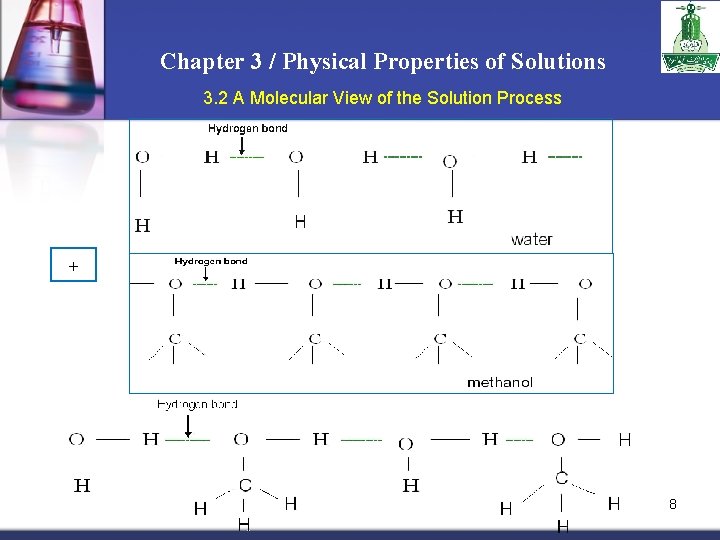
Chapter 3 / Physical Properties of Solutions 3. 2 A Molecular View of the Solution Process + 8

Chapter 3 / Physical Properties of Solutions 3. 3 Concentration Units n n The concentration of a solution is the amount of solute present in a given quantity of solvent or solution. Chemists use several different concentration units, Let us examine the four most common units of concentration: percent by mass, mole fraction, molarity, and molality. Percent by Mass % by mass = = mass of solute + mass of solvent mass of solute mass of solution x 100% 9

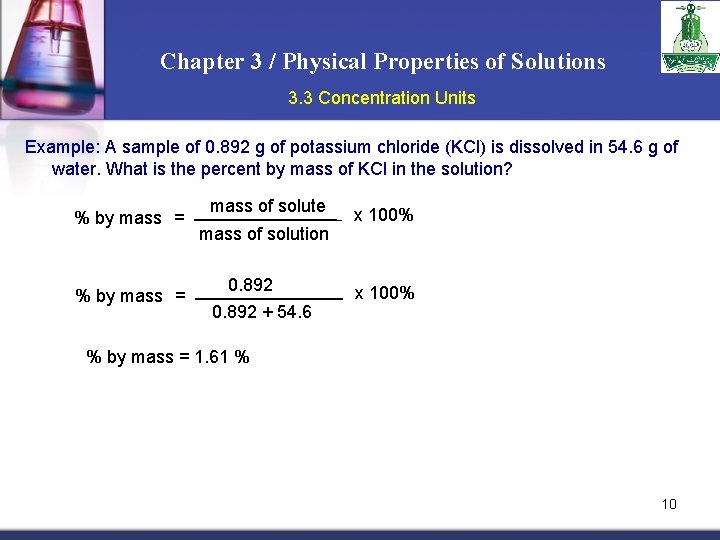
Chapter 3 / Physical Properties of Solutions 3. 3 Concentration Units Example: A sample of 0. 892 g of potassium chloride (KCl) is dissolved in 54. 6 g of water. What is the percent by mass of KCl in the solution? % by mass = mass of solute mass of solution 0. 892 + 54. 6 x 100% % by mass = 1. 61 % 10

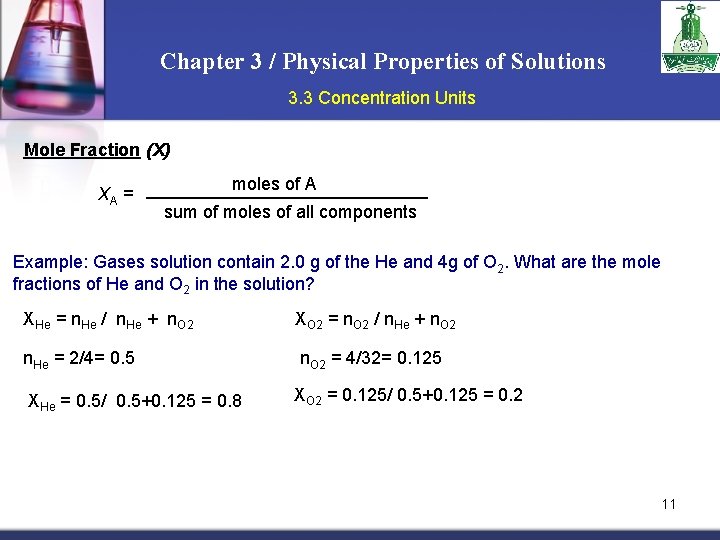
Chapter 3 / Physical Properties of Solutions 3. 3 Concentration Units Mole Fraction (X) XA = moles of A sum of moles of all components Example: Gases solution contain 2. 0 g of the He and 4 g of O 2. What are the mole fractions of He and O 2 in the solution? XHe = n. He / n. He + n. O 2 XO 2 = n. O 2 / n. He + n. O 2 n. He = 2/4= 0. 5 n. O 2 = 4/32= 0. 125 XHe = 0. 5/ 0. 5+0. 125 = 0. 8 XO 2 = 0. 125/ 0. 5+0. 125 = 0. 2 11

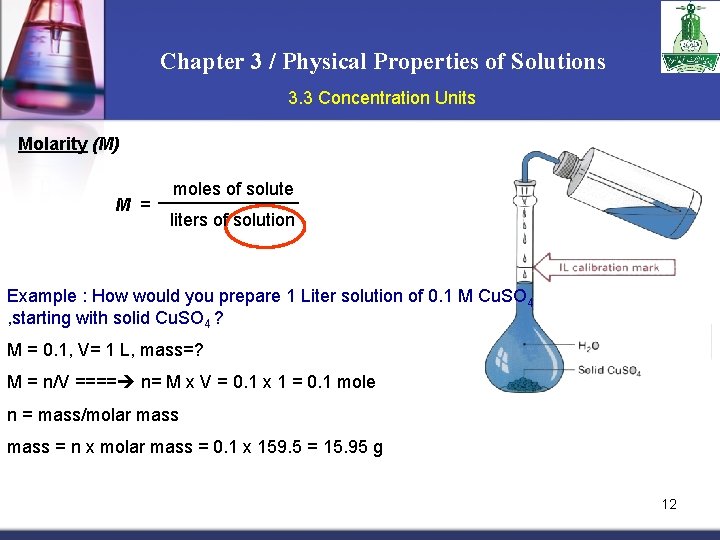
Chapter 3 / Physical Properties of Solutions 3. 3 Concentration Units Molarity (M) M = moles of solute liters of solution Example : How would you prepare 1 Liter solution of 0. 1 M Cu. SO 4 , starting with solid Cu. SO 4 ? M = 0. 1, V= 1 L, mass=? M = n/V ==== n= M x V = 0. 1 x 1 = 0. 1 mole n = mass/molar mass = n x molar mass = 0. 1 x 159. 5 = 15. 95 g 12

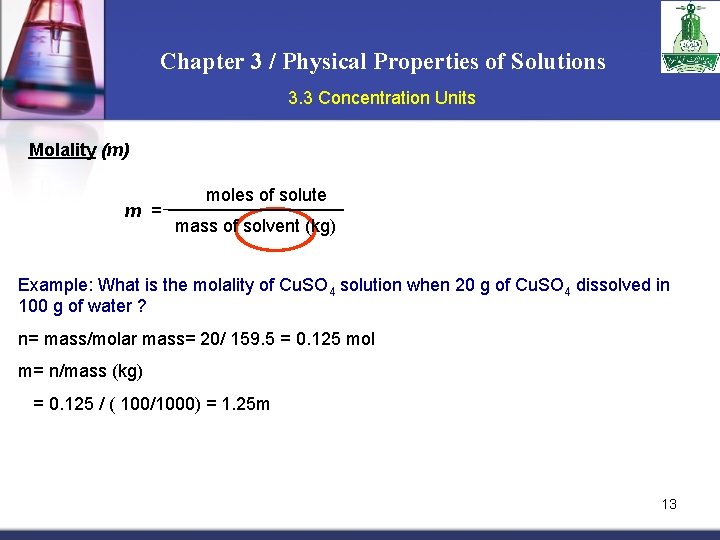
Chapter 3 / Physical Properties of Solutions 3. 3 Concentration Units Molality (m) m = moles of solute mass of solvent (kg) Example: What is the molality of Cu. SO 4 solution when 20 g of Cu. SO 4 dissolved in 100 g of water ? n= mass/molar mass= 20/ 159. 5 = 0. 125 mol m= n/mass (kg) = 0. 125 / ( 100/1000) = 1. 25 m 13

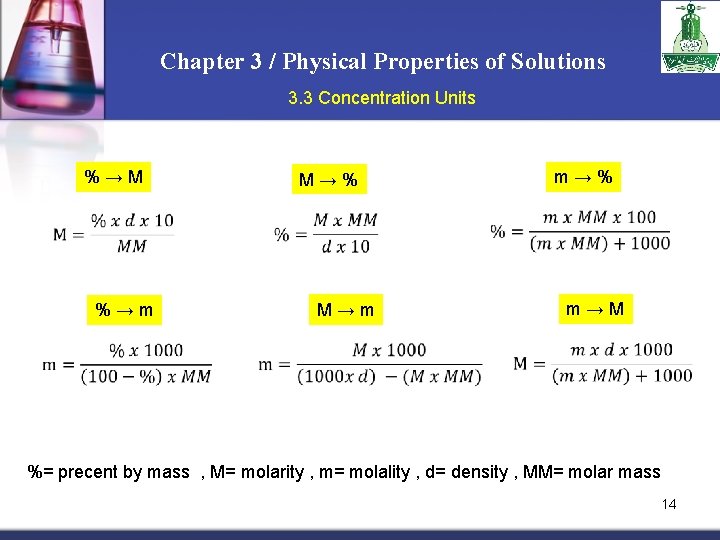
Chapter 3 / Physical Properties of Solutions 3. 3 Concentration Units % → M m → M M → m % → m m → % M → % %= precent by mass , M= molarity , m= molality , d= density , MM= molar mass 14

Chapter 3 / Physical Properties of Solutions 3. 3 Concentration Units Example: What is the molality of a 5. 86 M ethanol (C 2 H 5 OH) solution whose density is 0. 927 g/m. L? 15

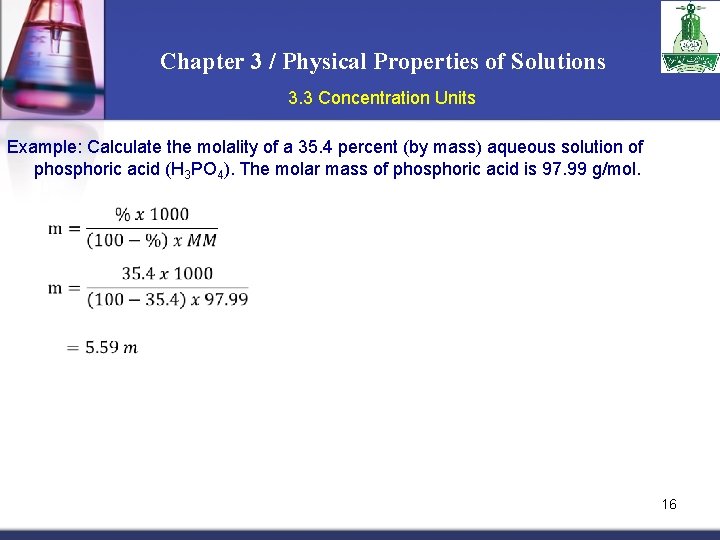
Chapter 3 / Physical Properties of Solutions 3. 3 Concentration Units Example: Calculate the molality of a 35. 4 percent (by mass) aqueous solution of phosphoric acid (H 3 PO 4). The molar mass of phosphoric acid is 97. 99 g/mol. 16

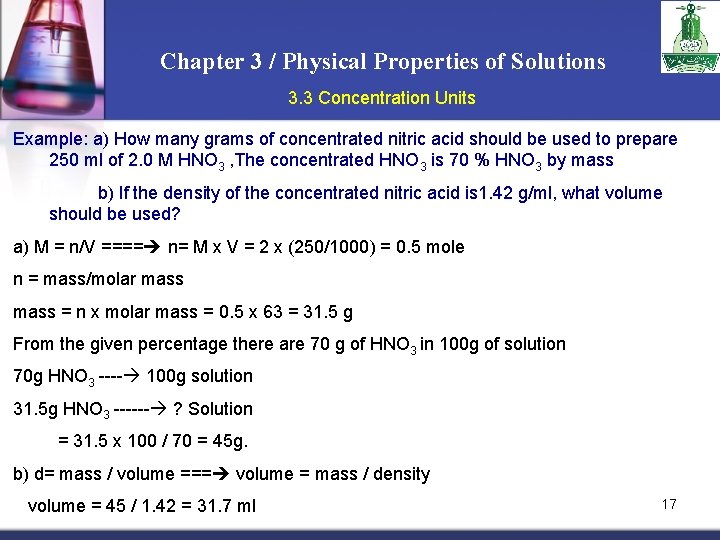
Chapter 3 / Physical Properties of Solutions 3. 3 Concentration Units Example: a) How many grams of concentrated nitric acid should be used to prepare 250 ml of 2. 0 M HNO 3 , The concentrated HNO 3 is 70 % HNO 3 by mass b) If the density of the concentrated nitric acid is 1. 42 g/ml, what volume should be used? a) M = n/V ==== n= M x V = 2 x (250/1000) = 0. 5 mole n = mass/molar mass = n x molar mass = 0. 5 x 63 = 31. 5 g From the given percentage there are 70 g of HNO 3 in 100 g of solution 70 g HNO 3 ---- 100 g solution 31. 5 g HNO 3 ------ ? Solution = 31. 5 x 100 / 70 = 45 g. b) d= mass / volume === volume = mass / density volume = 45 / 1. 42 = 31. 7 ml 17

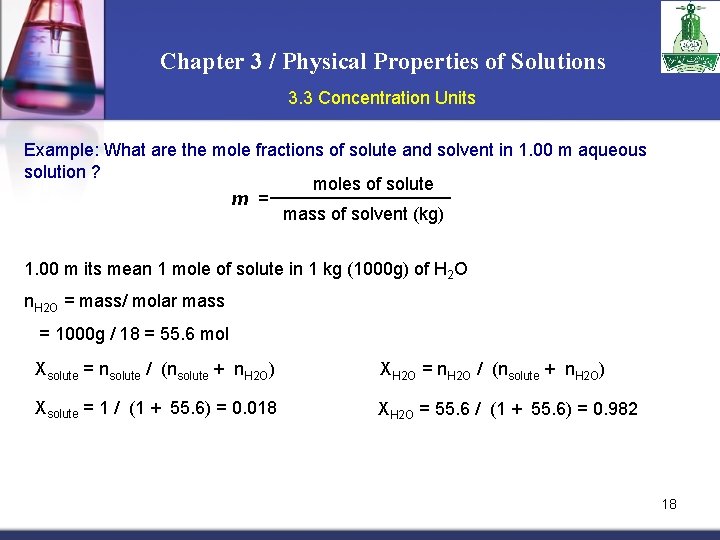
Chapter 3 / Physical Properties of Solutions 3. 3 Concentration Units Example: What are the mole fractions of solute and solvent in 1. 00 m aqueous solution ? moles of solute m = mass of solvent (kg) 1. 00 m its mean 1 mole of solute in 1 kg (1000 g) of H 2 O n. H 2 O = mass/ molar mass = 1000 g / 18 = 55. 6 mol Xsolute = nsolute / (nsolute + n. H 2 O) Xsolute = 1 / (1 + 55. 6) = 0. 018 XH 2 O = n. H 2 O / (nsolute + n. H 2 O) XH 2 O = 55. 6 / (1 + 55. 6) = 0. 982 18

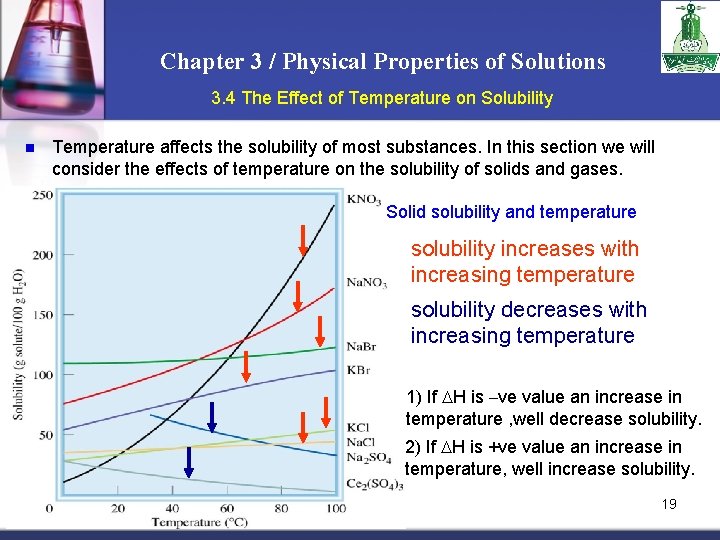
Chapter 3 / Physical Properties of Solutions 3. 4 The Effect of Temperature on Solubility n Temperature affects the solubility of most substances. In this section we will consider the effects of temperature on the solubility of solids and gases. Solid solubility and temperature solubility increases with increasing temperature solubility decreases with increasing temperature 1) If H is –ve value an increase in temperature , well decrease solubility. 2) If H is +ve value an increase in temperature, well increase solubility. 19

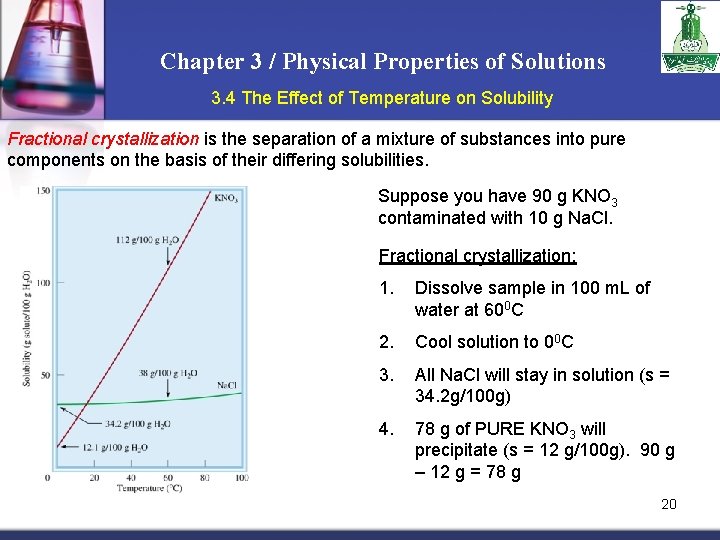
Chapter 3 / Physical Properties of Solutions 3. 4 The Effect of Temperature on Solubility Fractional crystallization is the separation of a mixture of substances into pure components on the basis of their differing solubilities. Suppose you have 90 g KNO 3 contaminated with 10 g Na. Cl. Fractional crystallization: 1. Dissolve sample in 100 m. L of water at 600 C 2. Cool solution to 00 C 3. All Na. Cl will stay in solution (s = 34. 2 g/100 g) 4. 78 g of PURE KNO 3 will precipitate (s = 12 g/100 g). 90 g – 12 g = 78 g 20

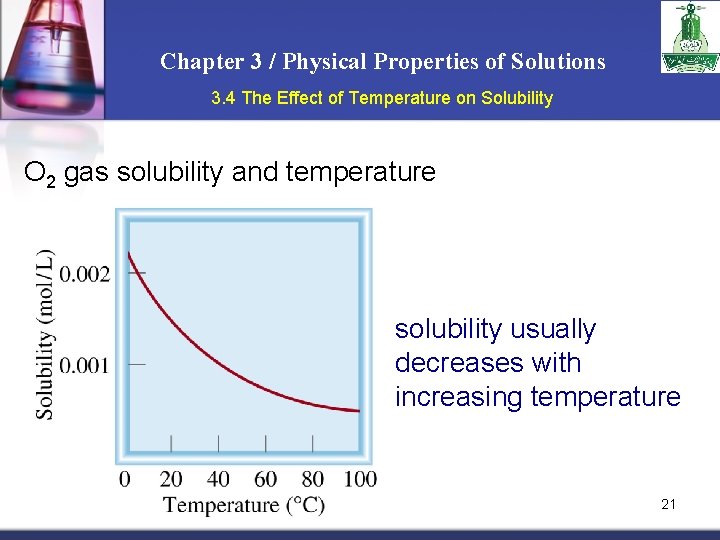
Chapter 3 / Physical Properties of Solutions 3. 4 The Effect of Temperature on Solubility O 2 gas solubility and temperature solubility usually decreases with increasing temperature 21

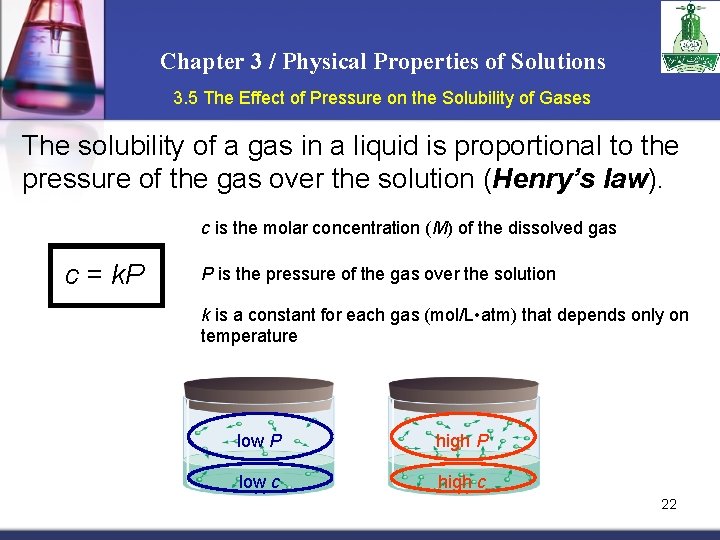
Chapter 3 / Physical Properties of Solutions 3. 5 The Effect of Pressure on the Solubility of Gases The solubility of a gas in a liquid is proportional to the pressure of the gas over the solution (Henry’s law). c is the molar concentration (M) of the dissolved gas c = k. P P is the pressure of the gas over the solution k is a constant for each gas (mol/L • atm) that depends only on temperature low P high P low c high c 22

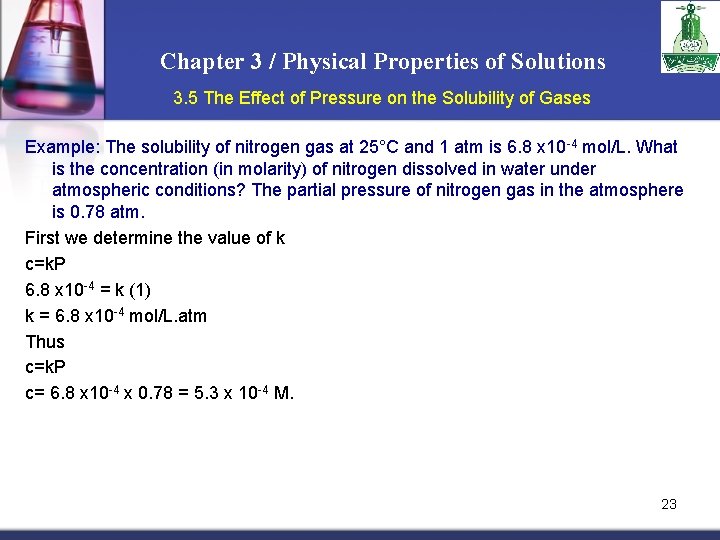
Chapter 3 / Physical Properties of Solutions 3. 5 The Effect of Pressure on the Solubility of Gases Example: The solubility of nitrogen gas at 25°C and 1 atm is 6. 8 x 10 -4 mol/L. What is the concentration (in molarity) of nitrogen dissolved in water under atmospheric conditions? The partial pressure of nitrogen gas in the atmosphere is 0. 78 atm. First we determine the value of k c=k. P 6. 8 x 10 -4 = k (1) k = 6. 8 x 10 -4 mol/L. atm Thus c=k. P c= 6. 8 x 10 -4 x 0. 78 = 5. 3 x 10 -4 M. 23

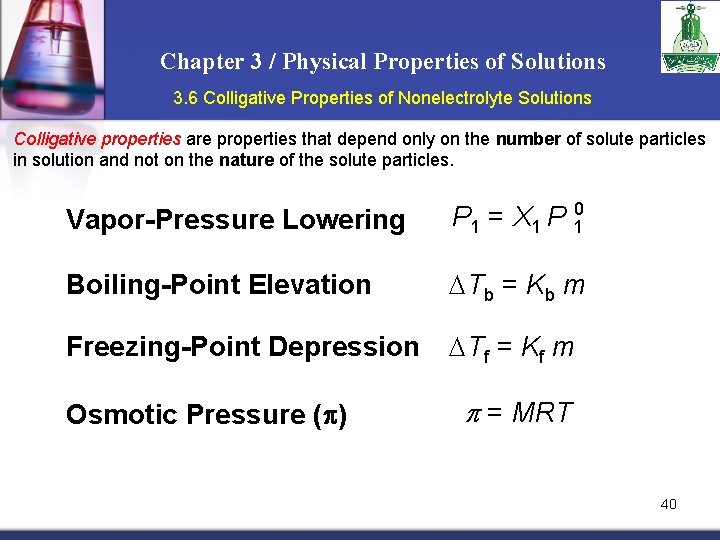
Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions Colligative properties are properties that depend only on the number of solute particles in solution and not on the nature of the solute particles. The colligative properties are vapor-pressure lowering, boiling-point elevation, freezingpoint depression, and osmotic pressure. For our discussion of colligative properties of nonelectrolyte solutions it is important to keep in mind that we are talking about relatively dilute solutions, that is, solutions whose concentrations are ≤ 0. 2 M. Vapor-Pressure Lowering P 1 = X 1 P 0 1 Raoult’s law P 01 = vapor pressure of pure solvent X 1 = mole fraction of the solvent If the solution contains only one solute: X 1 = 1 – X 2 P 10 - P 1 = P = X 2 P 10 X 2 = mole fraction of the solute 24

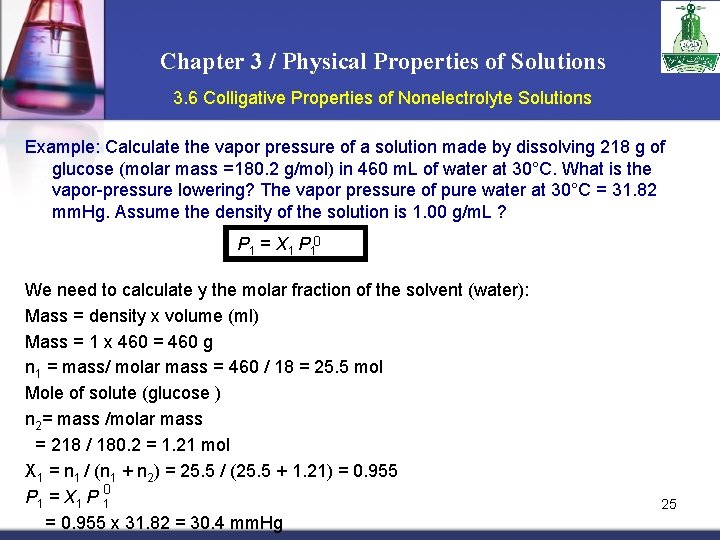
Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions Example: Calculate the vapor pressure of a solution made by dissolving 218 g of glucose (molar mass =180. 2 g/mol) in 460 m. L of water at 30°C. What is the vapor-pressure lowering? The vapor pressure of pure water at 30°C = 31. 82 mm. Hg. Assume the density of the solution is 1. 00 g/m. L ? P 1 = X 1 P 10 We need to calculate y the molar fraction of the solvent (water): Mass = density x volume (ml) Mass = 1 x 460 = 460 g n 1 = mass/ molar mass = 460 / 18 = 25. 5 mol Mole of solute (glucose ) n 2= mass /molar mass = 218 / 180. 2 = 1. 21 mol X 1 = n 1 / (n 1 + n 2) = 25. 5 / (25. 5 + 1. 21) = 0. 955 0 P 1 = X 1 P 1 = 0. 955 x 31. 82 = 30. 4 mm. Hg 25

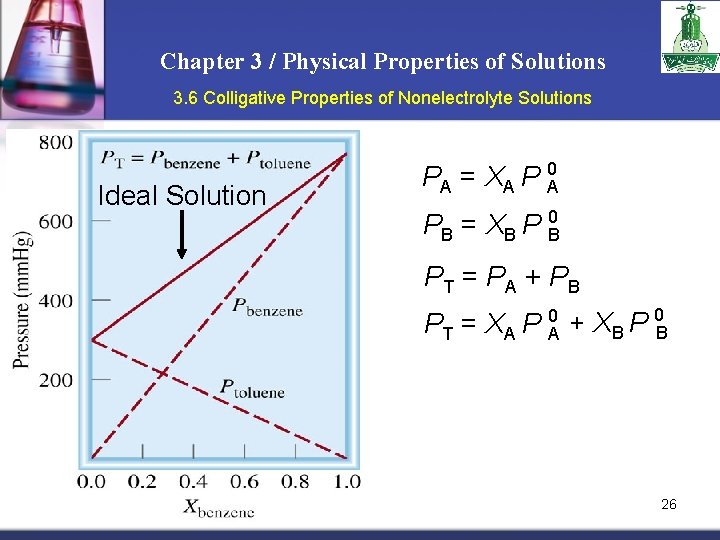
Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions Ideal Solution PA = XA P 0 A PB = XB P 0 B PT = PA + PB PT = XA P A 0 + XB P 0 B 26

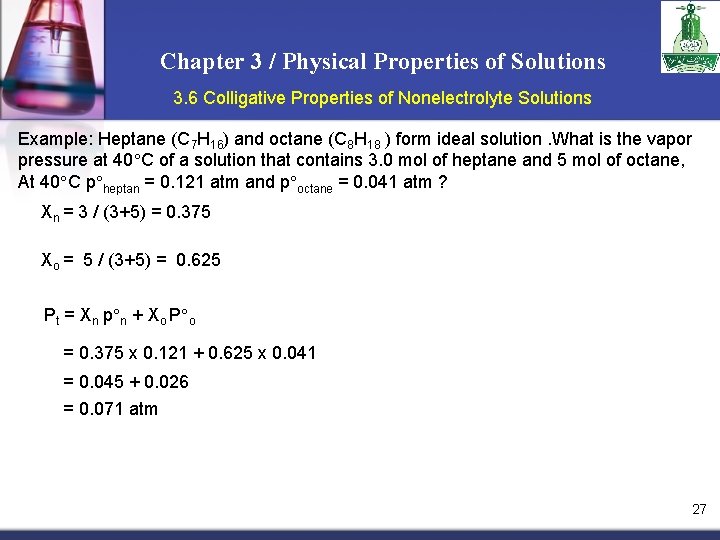
Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions Example: Heptane (C 7 H 16) and octane (C 8 H 18 ) form ideal solution. What is the vapor pressure at 40 C of a solution that contains 3. 0 mol of heptane and 5 mol of octane, At 40 C p heptan = 0. 121 atm and p octane = 0. 041 atm ? Xn = 3 / (3+5) = 0. 375 Xo = 5 / (3+5) = 0. 625 Pt = Xn p n + Xo P o = 0. 375 x 0. 121 + 0. 625 x 0. 041 = 0. 045 + 0. 026 = 0. 071 atm 27

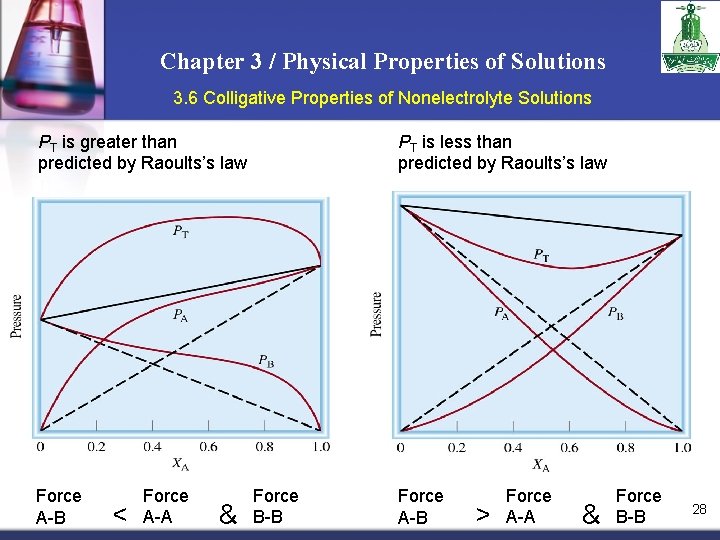
Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions PT is greater than predicted by Raoults’s law Force A-B < Force A-A & PT is less than predicted by Raoults’s law Force B-B Force A-B > Force A-A & Force B-B 28

Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions Fractional Distillation Apparatus 29

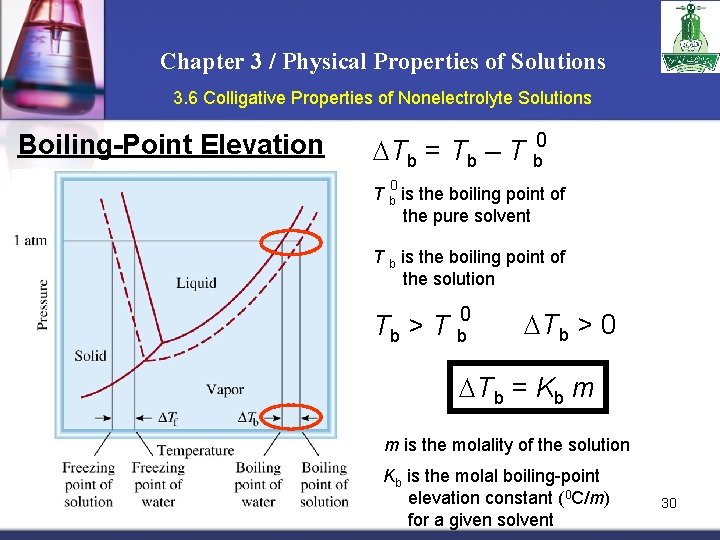
Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions Boiling-Point Elevation 0 Tb = Tb – T b 0 T b is the boiling point of the pure solvent T b is the boiling point of the solution Tb > T b 0 Tb > 0 Tb = Kb m m is the molality of the solution Kb is the molal boiling-point elevation constant (0 C/m) for a given solvent 30

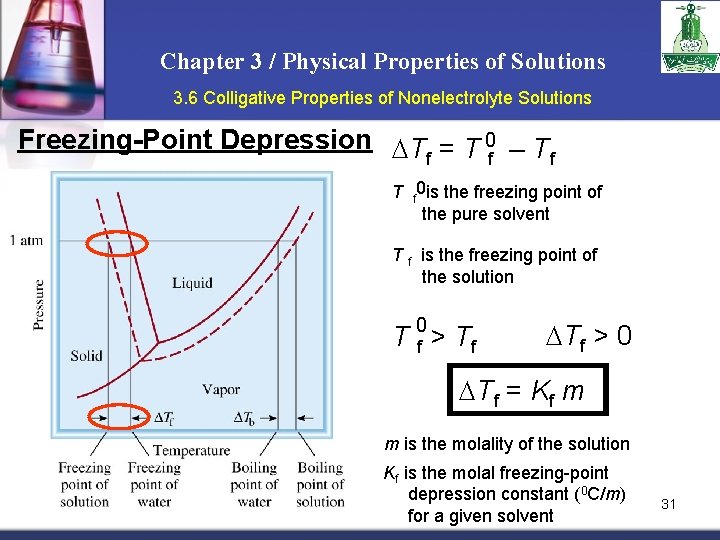
Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions Freezing-Point Depression T = T 0 – T f f f T f 0 is the freezing point of the pure solvent T f is the freezing point of the solution 0 T f > Tf > 0 Tf = Kf m m is the molality of the solution Kf is the molal freezing-point depression constant (0 C/m) for a given solvent 31

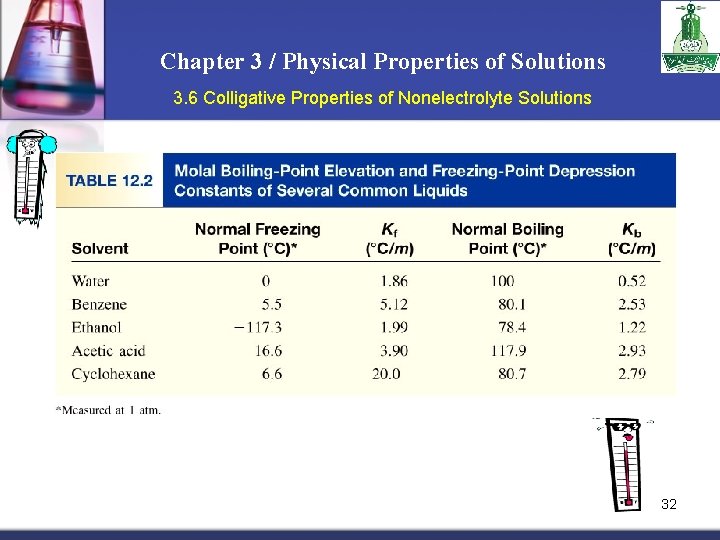
Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions 32

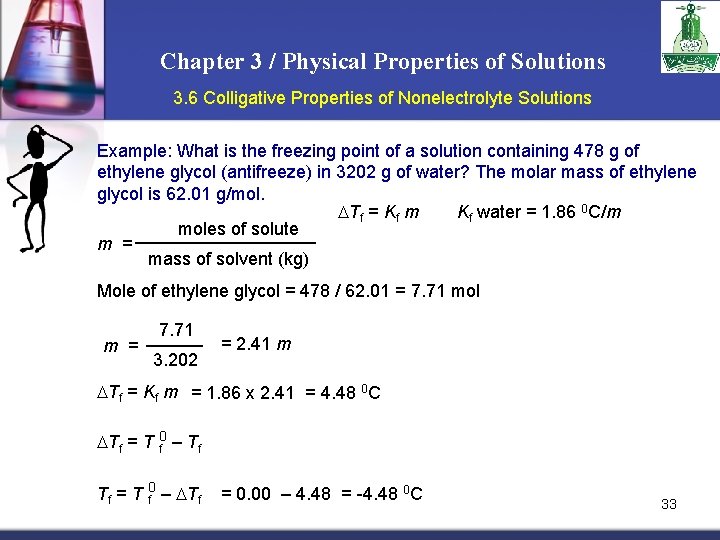
Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions Example: What is the freezing point of a solution containing 478 g of ethylene glycol (antifreeze) in 3202 g of water? The molar mass of ethylene glycol is 62. 01 g/mol. Tf = Kf m Kf water = 1. 86 0 C/m moles of solute m = mass of solvent (kg) Mole of ethylene glycol = 478 / 62. 01 = 7. 71 mol m = 7. 71 3. 202 = 2. 41 m Tf = Kf m = 1. 86 x 2. 41 = 4. 48 0 C Tf = T f 0 – Tf Tf = T 0 f – Tf = 0. 00 – 4. 48 = -4. 48 0 C 33

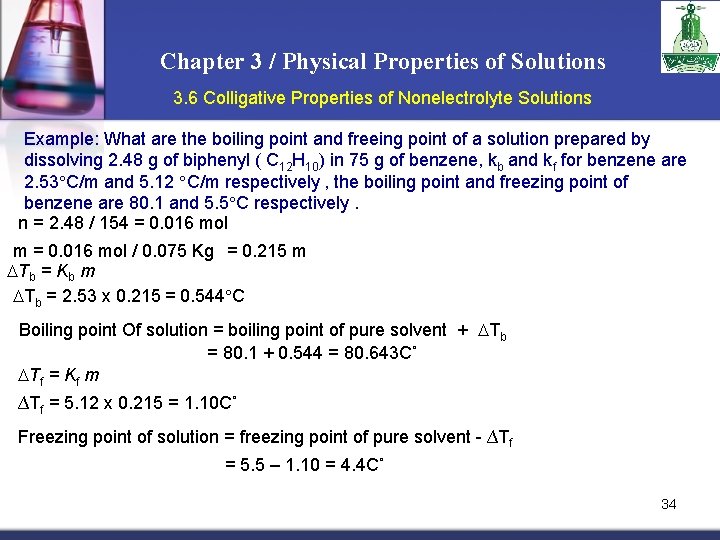
Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions Example: What are the boiling point and freeing point of a solution prepared by dissolving 2. 48 g of biphenyl ( C 12 H 10) in 75 g of benzene, kb and kf for benzene are 2. 53 C/m and 5. 12 C/m respectively , the boiling point and freezing point of benzene are 80. 1 and 5. 5 C respectively. n = 2. 48 / 154 = 0. 016 mol m = 0. 016 mol / 0. 075 Kg = 0. 215 m Tb = Kb m Tb = 2. 53 x 0. 215 = 0. 544 C Boiling point Of solution = boiling point of pure solvent + Tb = 80. 1 + 0. 544 = 80. 643 C˚ Tf = Kf m ∆Tf = 5. 12 x 0. 215 = 1. 10 C˚ Freezing point of solution = freezing point of pure solvent - ∆Tf = 5. 5 – 1. 10 = 4. 4 C˚ 34

Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions Example: A 7. 85 g sample of a compound is dissolved in 301 g of benzene. The freezing point of the solution is 1. 05°C below that of pure benzene. What are the molar mass of this compound? Tf = Kf m Kf benzene = 5. 12 0 C/m m = Tf / Kf = 1. 05 / 5. 12 = 0. 205 mol/kg m = moles of solute mass of solvent (kg) Mole = molality x mass of solvent (kg) = 0. 205 x 0. 301 = 0. 0617 mol Mole = mass (g) / molar mass Molar mass = mass / mole = 7. 85 / 0. 0617 = 127 g/mol 35

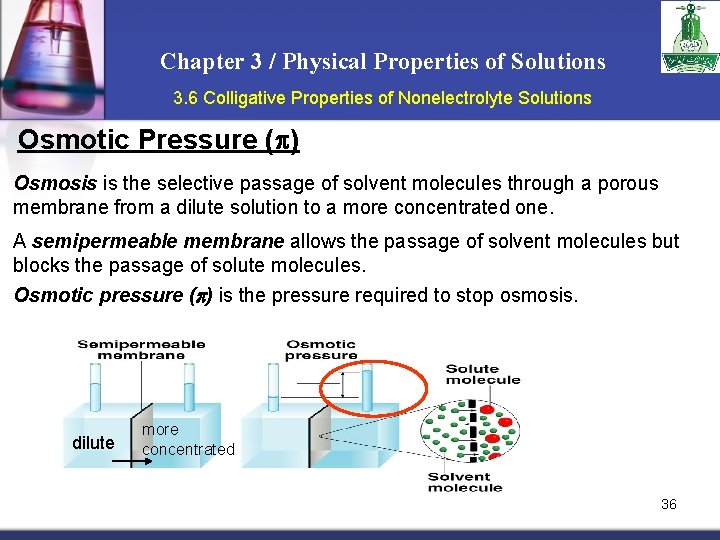
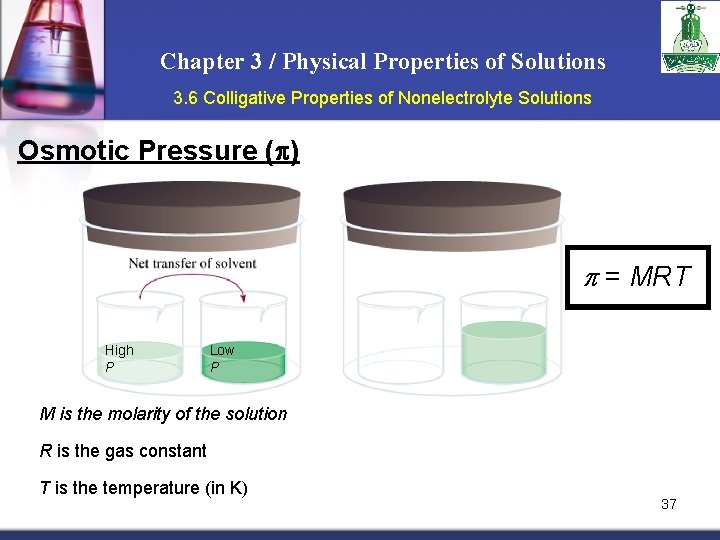
Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions Osmotic Pressure (p) Osmosis is the selective passage of solvent molecules through a porous membrane from a dilute solution to a more concentrated one. A semipermeable membrane allows the passage of solvent molecules but blocks the passage of solute molecules. Osmotic pressure (p) is the pressure required to stop osmosis. dilute more concentrated 36

Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions Osmotic Pressure (p) p = MRT High P Low P M is the molarity of the solution R is the gas constant T is the temperature (in K) 37

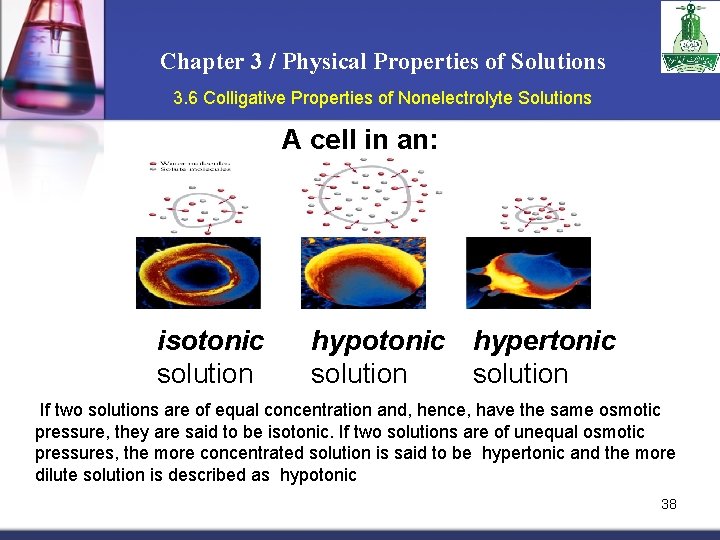
Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions A cell in an: isotonic solution hypotonic hypertonic solution If two solutions are of equal concentration and, hence, have the same osmotic pressure, they are said to be isotonic. If two solutions are of unequal osmotic pressures, the more concentrated solution is said to be hypertonic and the more dilute solution is described as hypotonic 38

Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions Example: The average osmotic pressure of seawater is about 30. 0 atm at 25°C. Calculate the molar concentration of an aqueous solution of sucrose (C 12 H 22 O 11) that is isotonic with seawater? p = MRT M = p / (RT) = 30 / ( 0. 0821 X 298) = 1. 23 M 39

Chapter 3 / Physical Properties of Solutions 3. 6 Colligative Properties of Nonelectrolyte Solutions Colligative properties are properties that depend only on the number of solute particles in solution and not on the nature of the solute particles. Vapor-Pressure Lowering P 1 = X 1 P 10 Boiling-Point Elevation Tb = Kb m Freezing-Point Depression Tf = Kf m Osmotic Pressure (p) p = MRT 40

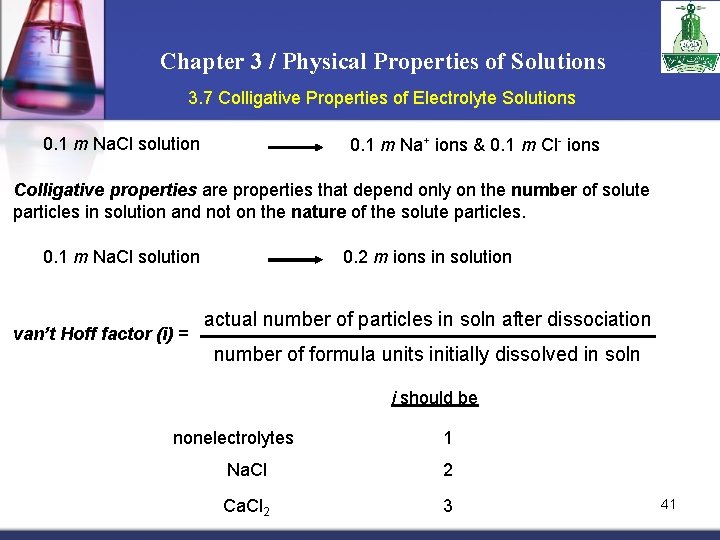
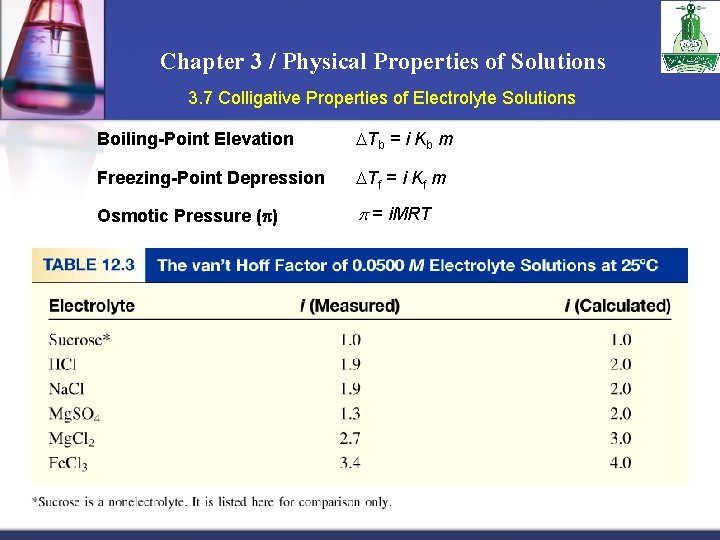
Chapter 3 / Physical Properties of Solutions 3. 7 Colligative Properties of Electrolyte Solutions 0. 1 m Na. Cl solution 0. 1 m Na+ ions & 0. 1 m Cl- ions Colligative properties are properties that depend only on the number of solute particles in solution and not on the nature of the solute particles. 0. 1 m Na. Cl solution van’t Hoff factor (i) = 0. 2 m ions in solution actual number of particles in soln after dissociation number of formula units initially dissolved in soln i should be nonelectrolytes 1 Na. Cl 2 Ca. Cl 2 3 41

Chapter 3 / Physical Properties of Solutions 3. 7 Colligative Properties of Electrolyte Solutions Boiling-Point Elevation Tb = i Kb m Freezing-Point Depression Tf = i Kf m Osmotic Pressure (p) p = i. MRT 42

Chapter 3 / Physical Properties of Solutions 3. 7 Colligative Properties of Electrolyte Solutions Example: The osmotic pressure of a 0. 010 M potassium iodide (KI) solution at 25°C is 0. 465 atm. Calculate the van’t Hoff factor for KI at this concentration? p = i. MRT i = p / (MRT) = 0. 465 / ( 0. 01 X 0. 0821 X 298) = 1. 90 43

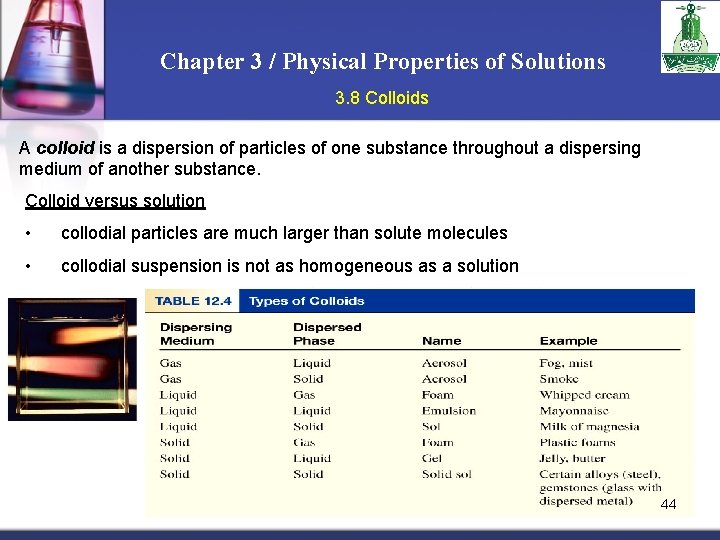
Chapter 3 / Physical Properties of Solutions 3. 8 Colloids A colloid is a dispersion of particles of one substance throughout a dispersing medium of another substance. Colloid versus solution • collodial particles are much larger than solute molecules • collodial suspension is not as homogeneous as a solution 44

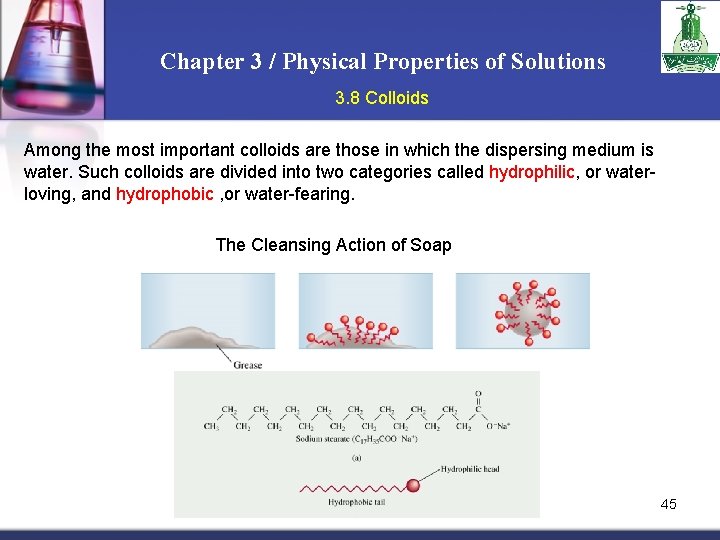
Chapter 3 / Physical Properties of Solutions 3. 8 Colloids Among the most important colloids are those in which the dispersing medium is water. Such colloids are divided into two categories called hydrophilic, or waterloving, and hydrophobic , or water-fearing. The Cleansing Action of Soap 45

Chapter 3 / Physical Properties of Solutions Thank you for listening 46