Physical Properties Chapter 2 Section 2 Physical Properties


















- Slides: 24

Physical Properties Chapter 2 Section 2

Physical Properties � Physical Property – any characteristic of a material that can be observed or measured without changing the composition of the substances in the materials. ◦ ◦ ◦ ◦ Viscosity Conductivity Malleability Hardness Melting point Boiling point Density

Viscosity � Resistance to flowing ◦ The greater the viscosity = slower the liquid moves ◦ Thin liquids have low viscosity �Water �Vinegar ◦ Thick liquids have high viscosity �Honey �Corn Syrup

Viscosity � Why usually decreases when it is heated is viscosity important?

Conductivity �A material’s ability to allow heat to flow � Materials with high conductivity such as metals are called conductors � If a material is a good conductor of heat it is usually a good conductor of electricity

Malleability � The ability of a solid to be hammered without shattering. � Most metals are malleable.

Hardness � Compare the hardness of two materials by seeing which material can scratch the other. � Diamond is the hardest known material.

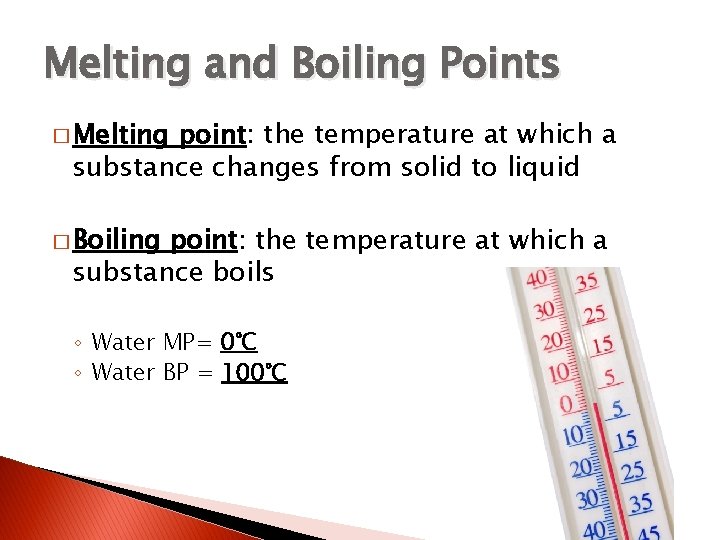
Melting and Boiling Points � Melting point: the temperature at which a substance changes from solid to liquid � Boiling point: the temperature at which a substance boils ◦ Water MP= 0°C ◦ Water BP = 100°C

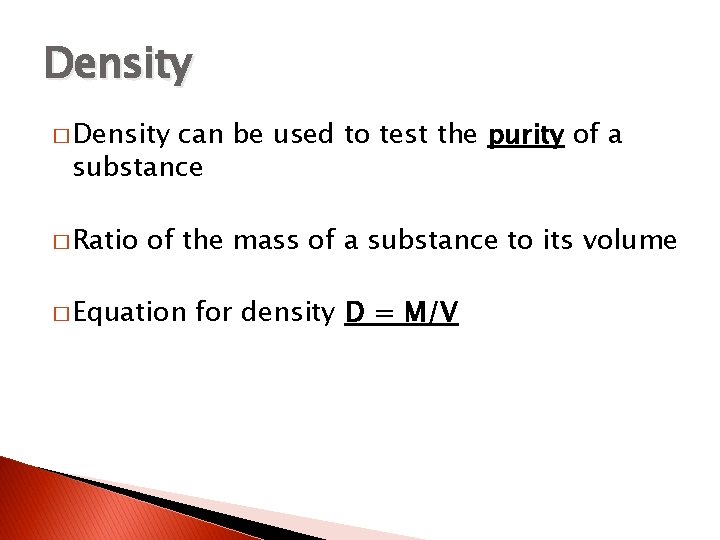
Density � Density can be used to test the purity of a substance � Ratio of the mass of a substance to its volume � Equation for density D = M/V

Using Physical Properties � Physical properties are used to: ◦ Identify a material ◦ Choose a material for a specific purpose ◦ To separate the substances in a mixture

Separation Methods � Filtration – the process that separates materials based on the size of their particles � Distillation – a process that separates the substances in a solution based on their boiling points

Recognizing Physical Changes � Physical change – occurs when some of the properties of a material change but the substances in the material stay the same � Some physical changes can be reversed others cannot be reversed ◦ Examples of physical changes �Melting butter in a pan �Crumpling paper �Slicing a tomato �Braiding hair �Peeling an orange

Reviewing Concepts � List 5 examples of physical properties � Describe 3 uses of physical properties � Name two procedures that are used to separate mixtures � When you describe a liquid as thick, are you saying that it has high or low viscosity? � Explain why sharpening a pencil is a physical change. � What allows a mixture to be separated by distillation?

Chemical Properties Chapter 2 Section 3

Chemical Property � Any ability to produce a change in the composition of matter. � Can be observed only when the substances in a sample of matter are changing into different substances. � Examples: ◦ Flammability ◦ Reactivity

Flammability �A material’s ability to burn in the presence of oxygen. � Sometimes flammability is a desirable property (gasoline, firewood) � Other times flammability is not desirable (fabrics for pajamas)

Reactivity � How readily a substance combines chemically with other substances � Examples ◦ Oxygen – high reactivity ◦ Nitrogen – low reactivity

Chemical Change � Occurs when a substance reacts and forms one or more new substances � Three common types of evidence for a chemical change: 1. Change in color 2. Production of a gas 3. Formation of a precipitate

Change in Color � Examples: ◦ ◦ ◦ Leaves changing color in the fall Ripening of a banana peel Silver bracelet exposed to air darkens Match that burns turns black Copper changing when exposed to moist air (patina)

Change in Color A new copper roof has a reddish color An old copper roof has a greenish color

Production of a Gas � Carbon dioxide gas forms when vinegar is mixed with baking soda � Baking powder in a cake causes a cake to rise ◦ Baking soda and acids react when wet, cake bakes, bubbles of CO 2 expand, cake rises

Formation of a Precipitate � Precipitate – any solid that forms and separates from a liquid mixture � Example: curds in cottage cheese – form when an acid is added to milk

Is a Change Chemical or Physical? � Are different substances present after the change takes place? ◦ NO – change is PHYSICAL ◦ YES – change is CHEMICAL ◦ When matter undergoes a chemical change the composition of matter changes. ◦ When matter undergoes a physical change the composition of matter remains the same.

Reviewing Concepts � List three common types of evidence for a chemical change. � How do chemical changes differ from physical changes? � Gold and platinum are often used to make jewelry. What can you infer about the reactivity of these elements? � A piece of butter melts and then burns in a hot frying pan. Which of these changes is physical and which is chemical?