Changes of State Chapter 10 Section 4 Review



















- Slides: 29

Changes of State Chapter 10 Section 4

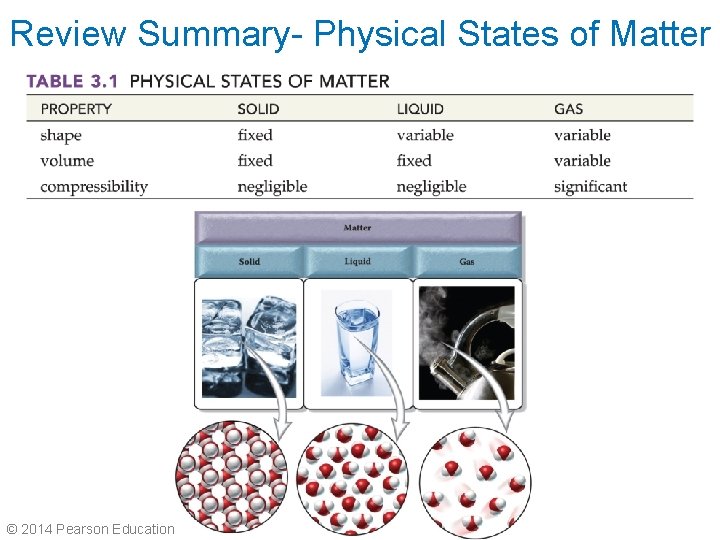
Review Summary- Physical States of Matter © 2014 Pearson Education, Inc. Chapter 3

Vocabulary • Phase: any part of a system that has uniform composition and properties • Condensation: the process by which a gas changes to a liquid • Equilibrium: a dynamic condition in which two opposing changes occur at equal rates in a closed system

More Information • Vapor: a gas in contact with its liquid or solid phase


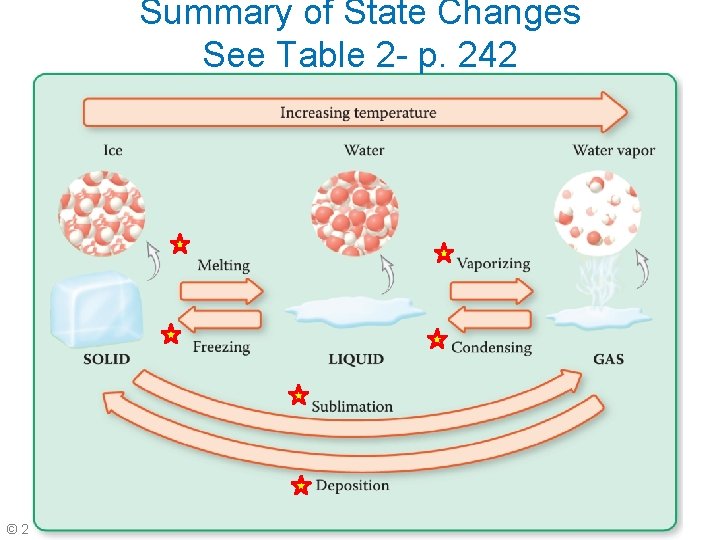
Summary of State Changes See Table 2 - p. 242 © 2014 Pearson Education, Inc. Chapter 3

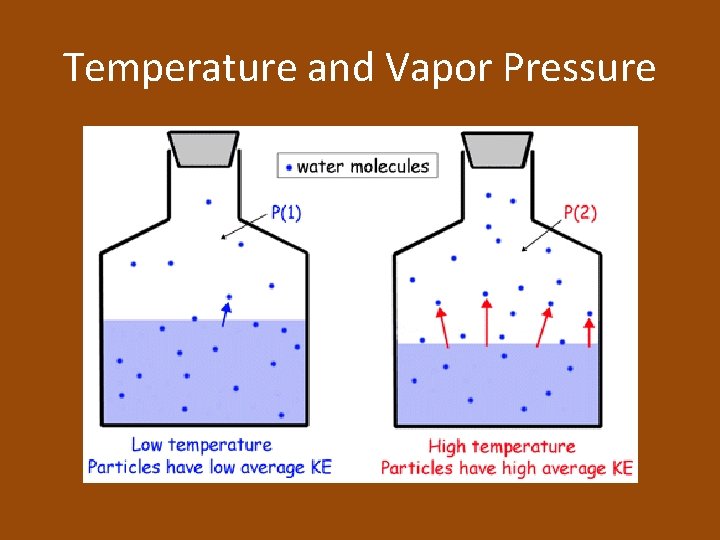
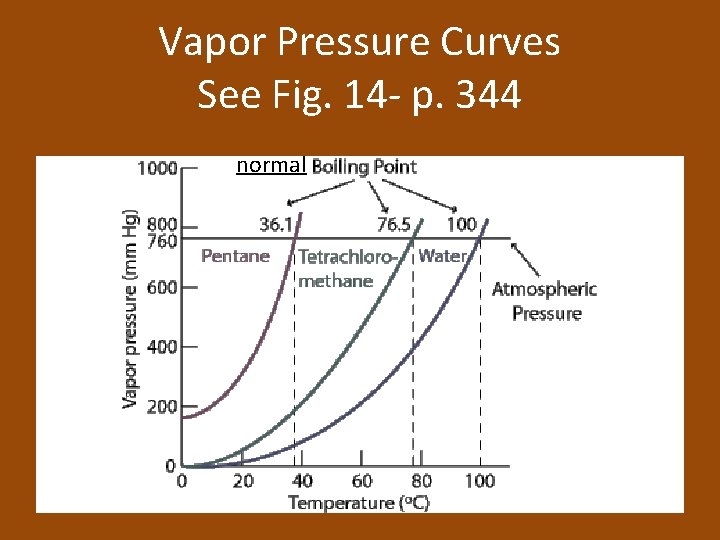
Equilibrium Vapor Pressure of a Liquid • Equilibrium vapor pressure: the pressure exerted by a vapor in equilibrium with its corresponding liquid at a given temperature • Increase temperature = increase in kinetic energy = increase in the number of molecules with enough energy to become a vapor = increase in equilibrium vapor pressure

At a Given Temperature • Because all liquids have characteristic forces of attraction between their particles, every liquid has a specific equilibrium vapor pressure at a given temperature • Stronger forces = smaller number of particles that can evaporate = low equilibrium vapor pressure

Equilibrium Vapor Pressure

Temperature and Vapor Pressure

Relative Equilibrium Vapor Pressure • Volatile liquids: liquids that evaporate readily • Volatile liquids have weak forces of attraction between their particles. – Example: Ether, acetone, gasoline, methanol • Nonvolatile liquids: liquids that do not evaporate readily

• Nonvolatile liquids have relatively strong attractive forces between their particles • Molten ionic compounds would be nonvolatile liquids because of the strong attraction for positive ions and negative ions in the liquid phase.

Boiling • Boiling allows the conversion of a liquid to a vapor within the liquid as well as at its surface. • Boiling point: the temperature at which the equilibrium vapor pressure of a liquid equals the atmospheric pressure • Energy must be added continuously for a liquid to keep boiling

Vapor Pressure Curves See Fig. 14 - p. 344 normal

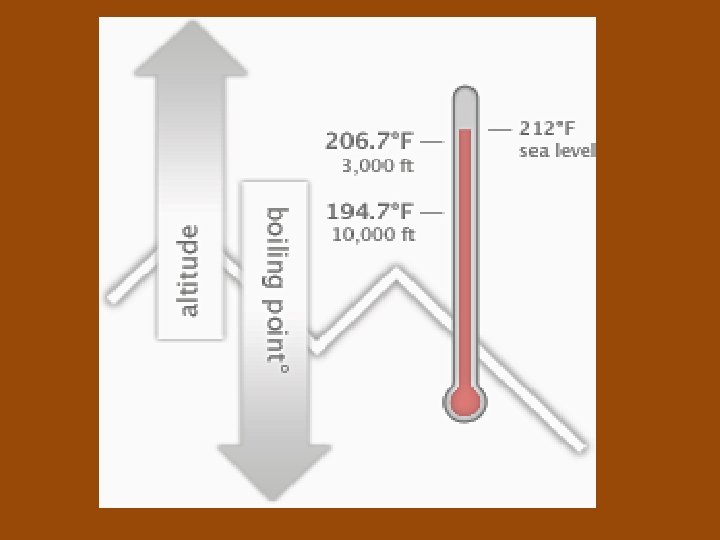
Pressure and Boiling Point • If you increase the pressure, then the boiling point is higher. • If you decrease the pressure, then the boiling point is lower. – At high altitudes, the atmospheric pressure is lower and water boils at a lower temperature, so it is recommended to bake at a higher temperature for less time. Some foods would require longer to cook.


• The temperature at the boiling point remains constant despite the continuous addition of energy. • The added energy is used to overcome the attractive forces between the particles in the liquid as it vaporizes. • This energy is stored in the vapor as potential energy.

Molar Enthalpy of Vaporization • Molar enthalpy of vaporization: the amount of energy as heat required to evaporate 1 mole of a liquid at constant pressure and temperature • Abbreviated as • This is actually a measure of the strength of the attractive forces between particles • Each liquid has a characteristic molar enthalpy of vaporization


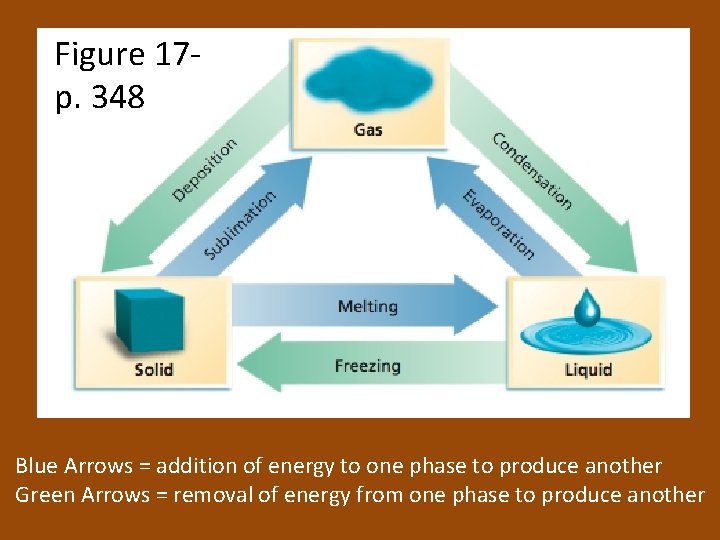
Freezing • Normal freezing point: the temperature at which the solid and liquid are in equilibrium at 1 atm (101. 3 k. Pa) – Particles of the liquid and the solid have the same average kinetic energy • Energy lost during freezing is the loss of stored energy (potential)

Melting is the reverse of freezing. It ocurs at a constant temperature. As a solid melts, it absorbs energy as heat. For pure crystalline solids, the melting point and the freezing point are the same. • At equilibrium, melting and freezing proceed at equal rates. • •

Molar Enthalpy of Fusion • Molar enthalpy of fusion: the amount of energy as heat required to melt one mole of solid at the solid’s melting point • Abbreviated as • The energy absorbed overcomes the attractive forces and decreases particle order (dependent on the strength of the forces).

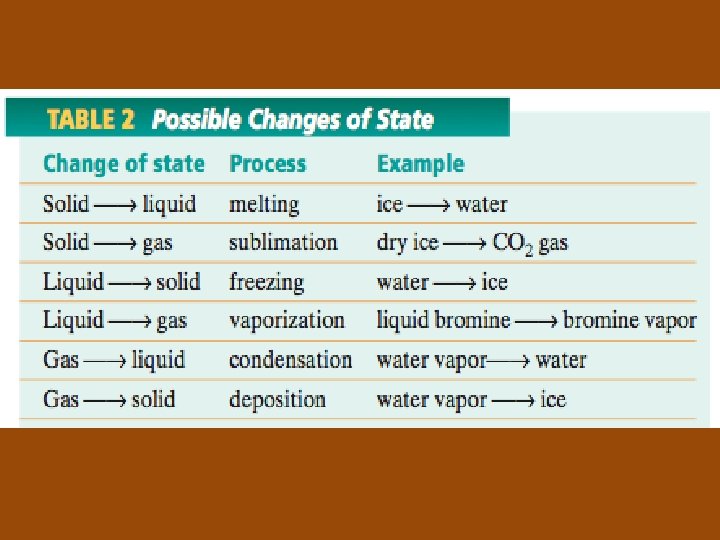
Sublimation and Deposition • Sublimation: the change of state from a solid directly to a gas • Examples: solid CO 2 and iodine • Deposition: the change of state from a gas directly to a solid • Example: formation of frost on a cold surface


Figure 17 p. 348 Blue Arrows = addition of energy to one phase to produce another Green Arrows = removal of energy from one phase to produce another

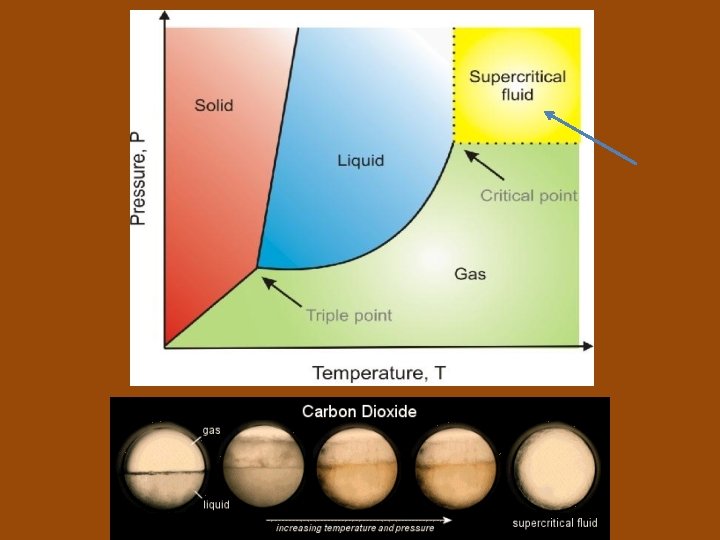
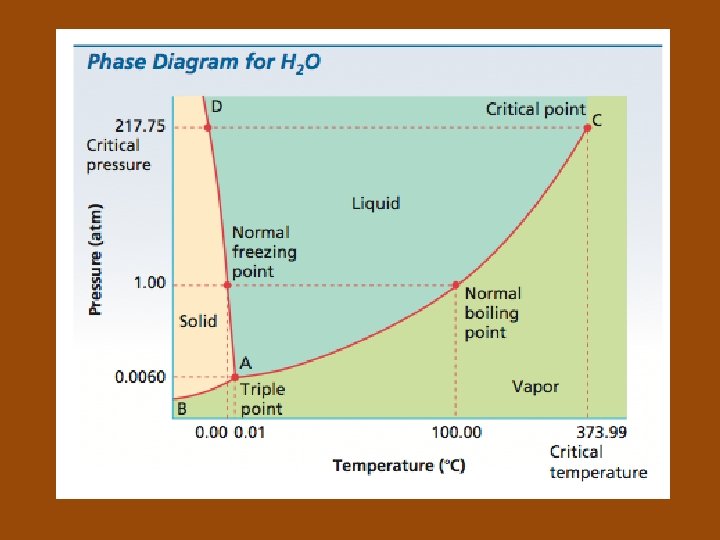
Phase Diagrams • Phase diagram: a graph of pressure versus temperature that shows the conditions under which the phases of a substance exist • Triple point (of a substance): indicates the temperature and pressure conditions at which the solid, liquid, and vapor states can coexist at equilibrium

• Critical point: of a substance, indicates the critical temperature and critical pressure • Critical temperature: the temperature above which the substance cannot exist in the liquid state • Critical pressure: the lowest pressure at which the substance can exist as a liquid at the critical temperature

