Chapter 13 Solutions 1 Solutions Recall Solutions are























- Slides: 45

Chapter 13 Solutions 1

Solutions • Recall: Solutions are homogeneous mixtures. ØAppears to be one substance, though really contains multiple materials. • Most homogeneous materials we encounter are actually solutions. ØE. g. , air and lake water. 2

Solutions, Continued • Solutions = Solute + Solvent • Solute is the dissolved substance. ØSeems to “disappear. ” Ø“Takes on the state” of the solvent. • Solvent is the substance solute dissolves in. ØDoes not appear to change state. ØE. g. Salt dissolved in water. 3

Solutions, Continued • When both solute and solvent have the same state, the solvent is the component present in the highest percentage. • E. g. mixture of 2 m. L of ethanol and 10 m. L of water. ØEthanol = solute ØWater = solvent 4

Types of Solutions • Saturated solutions have the maximum amount of solute that will dissolve in that solvent at that temperature. • Unsaturated solutions can dissolve more solute. • Supersaturated solutions are holding more solute than they should be able to. ØUnstable. 5

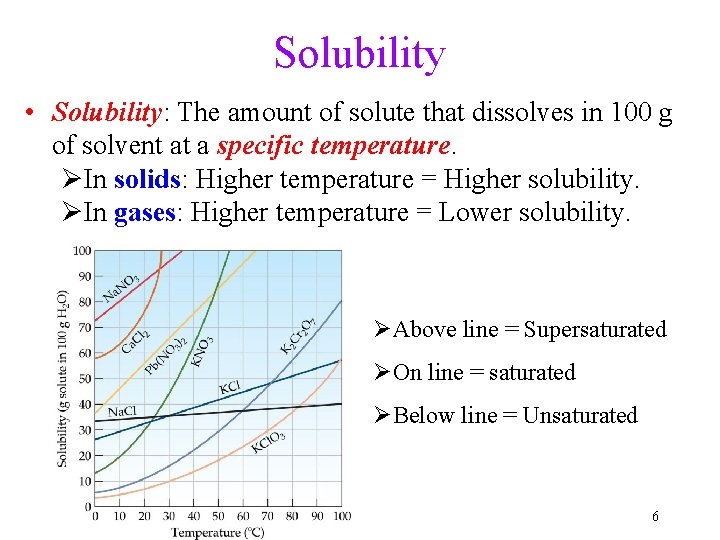
Solubility • Solubility: The amount of solute that dissolves in 100 g of solvent at a specific temperature. ØIn solids: Higher temperature = Higher solubility. ØIn gases: Higher temperature = Lower solubility. ØAbove line = Supersaturated ØOn line = saturated ØBelow line = Unsaturated 6

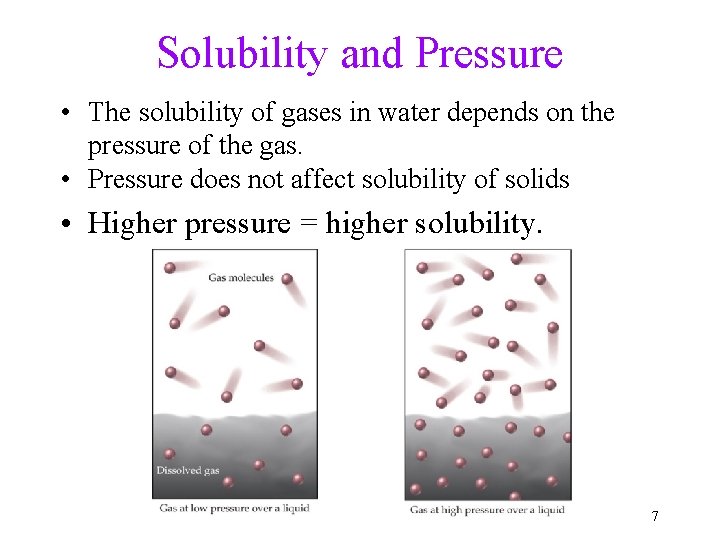
Solubility and Pressure • The solubility of gases in water depends on the pressure of the gas. • Pressure does not affect solubility of solids • Higher pressure = higher solubility. 7

Amount of Solute in a solution • Mostly determined by: ØMass percent ØConcentration (Molarity) 8

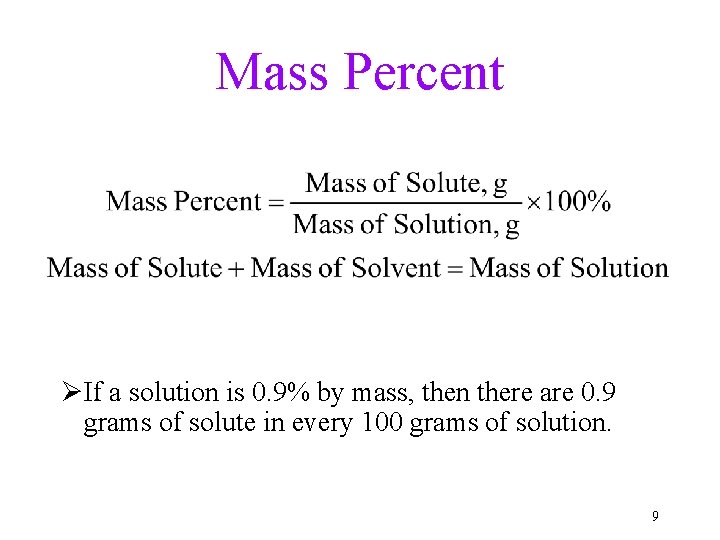
Mass Percent ØIf a solution is 0. 9% by mass, then there are 0. 9 grams of solute in every 100 grams of solution. 9

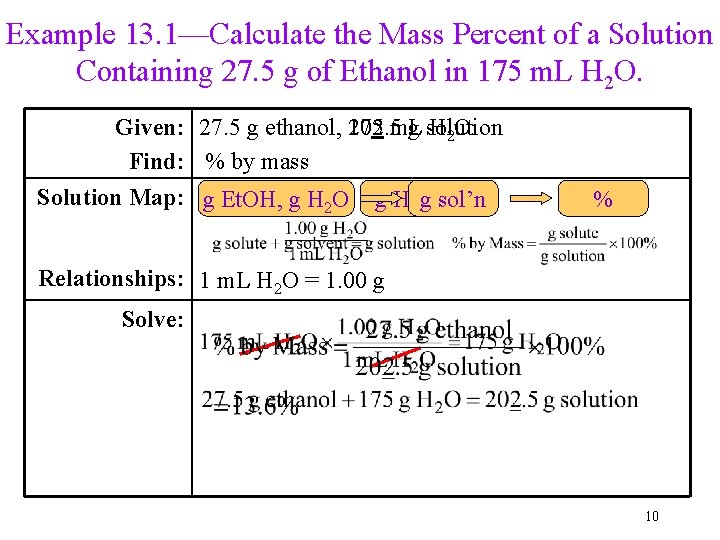
Example 13. 1—Calculate the Mass Percent of a Solution Containing 27. 5 g of Ethanol in 175 m. L H 2 O. Given: 27. 5 g ethanol, 202. 5 175 m. L g solution H 2 O Find: % by mass Solution Map: m. L g Et. OH, g sol’n H 2 O g H 2 O % Relationships: 1 m. L H 2 O = 1. 00 g Solve: 10

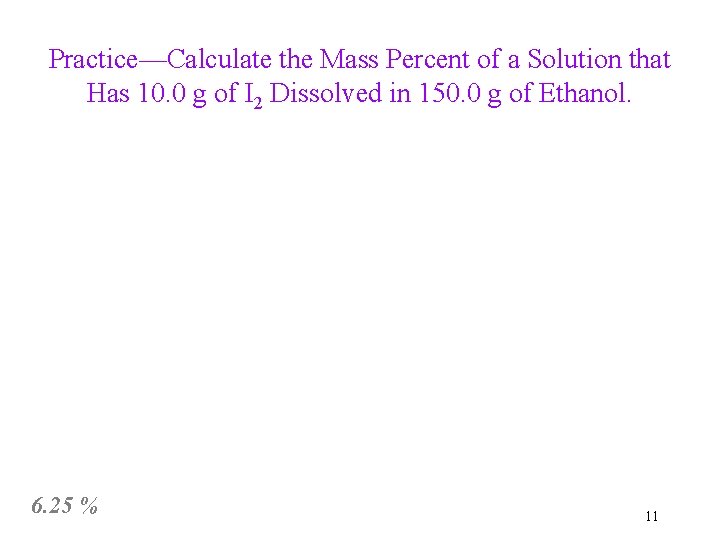
Practice—Calculate the Mass Percent of a Solution that Has 10. 0 g of I 2 Dissolved in 150. 0 g of Ethanol. 6. 25 % 11

Using Mass Percent as Conversion Factors Example—How Would You Prepare 250. 0 g of 5. 00% by Mass Glucose Solution ? 12

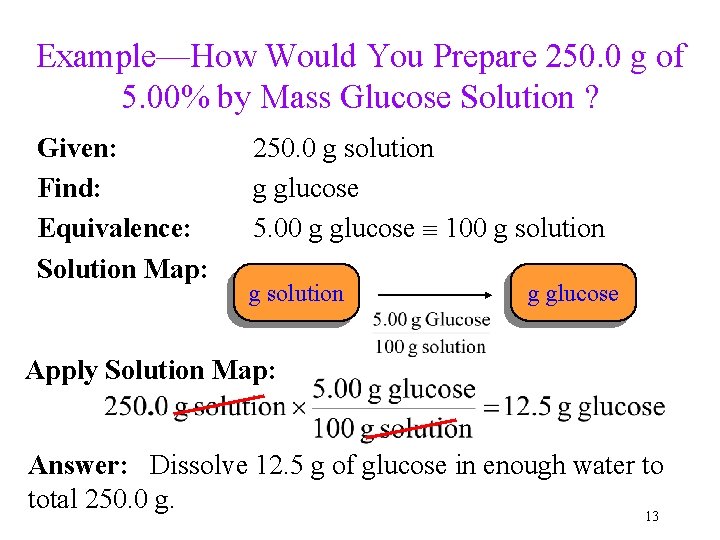
Example—How Would You Prepare 250. 0 g of 5. 00% by Mass Glucose Solution ? Given: Find: Equivalence: Solution Map: 250. 0 g solution g glucose 5. 00 g glucose 100 g solution g glucose Apply Solution Map: Answer: Dissolve 12. 5 g of glucose in enough water to total 250. 0 g. 13

Practice—Milk Is 4. 5% by Mass Lactose. Determine the Mass of Lactose in 175 g of Milk. 7. 9 g 14

Practice—How Would You Prepare 450. 0 g of 15. 0% by Mass Aqueous Ethanol? 67. 5 g in 382. 5 g water 15

Solution Concentrations also known as molarity • Concentration = Moles of solute in liters of solution. moles of solute Molarity = liters of solution • If a sugar solution concentration is 2. 0 M , Ø 1 liter of solution contains 2. 0 moles of sugar Ø 2 liters = 4. 0 moles sugar Ø 0. 5 liters = 1. 0 mole sugar 16

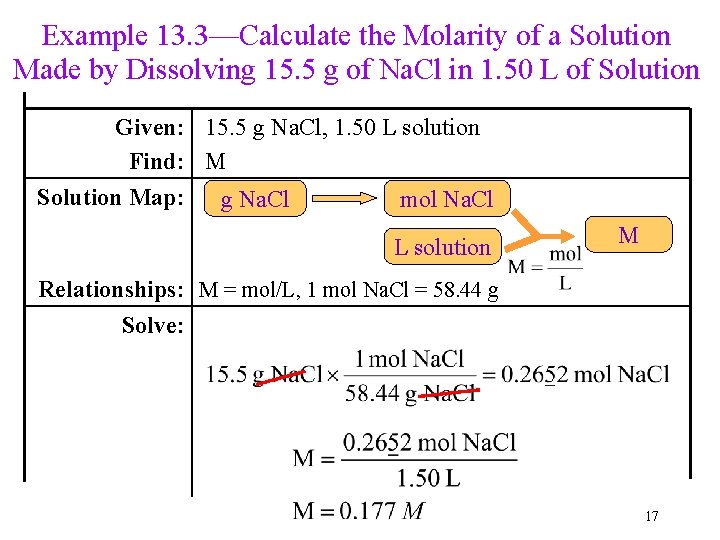
Example 13. 3—Calculate the Molarity of a Solution Made by Dissolving 15. 5 g of Na. Cl in 1. 50 L of Solution Given: 15. 5 g Na. Cl, 1. 50 L solution Find: M Solution Map: g Na. Cl mol Na. Cl L solution M Relationships: M = mol/L, 1 mol Na. Cl = 58. 44 g Solve: 17

Practice—What Is the Molarity of a Solution Containing 3. 4 g of NH 3 (MM 17. 03) in 200. 0 m. L of Solution? 1. 0 M 18

Practice—Determine the Mass of Ca. Cl 2 (MM = 110. 98) in 1. 75 L of 1. 50 M Solution 291 g 19

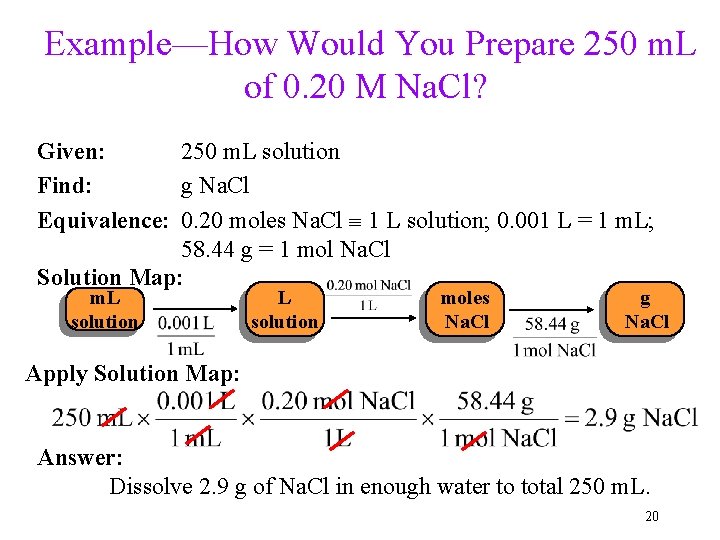
Example—How Would You Prepare 250 m. L of 0. 20 M Na. Cl? Given: 250 m. L solution Find: g Na. Cl Equivalence: 0. 20 moles Na. Cl 1 L solution; 0. 001 L = 1 m. L; 58. 44 g = 1 mol Na. Cl Solution Map: m. L solution moles Na. Cl g Na. Cl Apply Solution Map: Answer: Dissolve 2. 9 g of Na. Cl in enough water to total 250 m. L. 20

Practice—How Would You Prepare 100. 0 m. L of 0. 100 M K 2 SO 4 (MM = 174. 26)? 1. 74 g of K 2 SO 4 in enough water to total 100. 0 m. L. 21

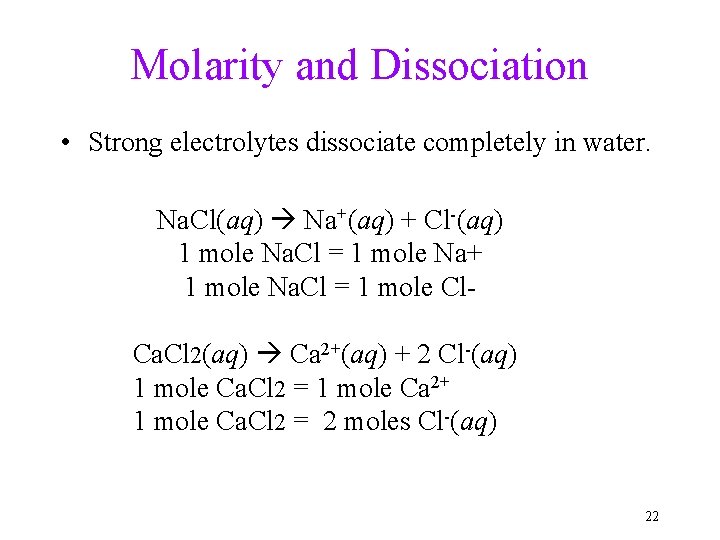
Molarity and Dissociation • Strong electrolytes dissociate completely in water. Na. Cl(aq) Na+(aq) + Cl-(aq) 1 mole Na. Cl = 1 mole Na+ 1 mole Na. Cl = 1 mole Cl. Ca. Cl 2(aq) Ca 2+(aq) + 2 Cl-(aq) 1 mole Ca. Cl 2 = 1 mole Ca 2+ 1 mole Ca. Cl 2 = 2 moles Cl-(aq) 22

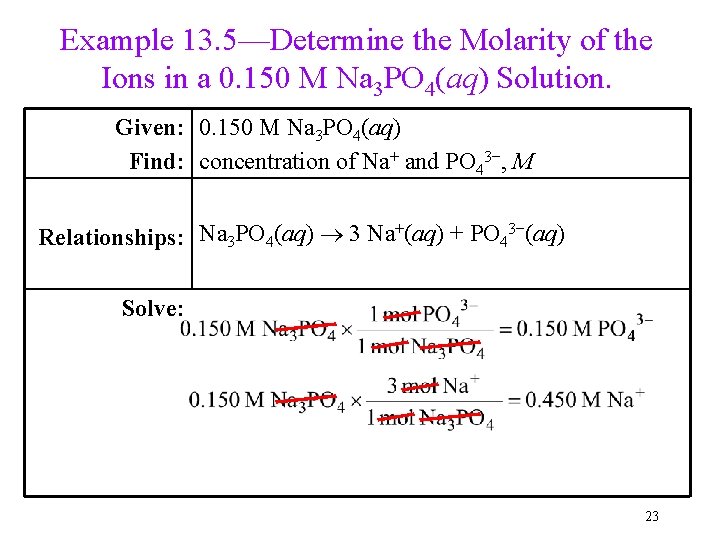
Example 13. 5—Determine the Molarity of the Ions in a 0. 150 M Na 3 PO 4(aq) Solution. Given: 0. 150 M Na 3 PO 4(aq) Find: concentration of Na+ and PO 43−, M Relationships: Na 3 PO 4(aq) 3 Na+(aq) + PO 43−(aq) Solve: 23

Practice—Find the Molarity of All Ions in the Given Solutions of Strong Electrolytes. • 0. 25 M Mg. Br 2(aq). • 0. 33 M Na 2 CO 3(aq). • 0. 0750 M Fe 2(SO 4)3(aq). 24

Dilution • Dilution is adding extra solvent to decrease the concentration of a solution. • The amount of solute stays the same, but the concentration decreases. • Dilution Formula: Concstart solnx Volstart soln = Concfinal solnx Volfinal sol • M 1 V 1 = M 2 V 2 25

*Example*—What Volume of 12. 0 M KCl Is Needed to Make 5. 00 L of 1. 50 M KCl Solution? 26

Example—Dilution Problems • What is the concentration of a solution made by diluting 15 m. L of 5. 0% sugar to 135 m. L? M 1 = 5. 0 % M 2 = ? % V 1 = 15 m. L V 2 = 135 m. L (5. 0%)(15 m. L) = M 2 x (135 m. L) M 2 = 0. 55% • How would you prepare 200 m. L of 0. 25 M Na. Cl solution from a 2. 0 M solution? M 1 = 2. 0 M V 1 = ? m. L M 2 = 0. 25 M V 2 = 200 m. L (2. 0 M) x V 1 = (0. 25 M)(200 m. L) V 1 = 25 m. L Dilute 25 m. L of 2. 0 M Na. Cl solution to 200 m. L. 27

Practice—Determine the Concentration of the Following Solutions. • Made by diluting 125 m. L of 0. 80 M HCl to 500 m. L. 0. 2 M 28

Practice Question 1—How Would You Prepare 400 m. L of a 4. 0% Solution From a 12% Solution? Practice Question 2—How Would You Prepare 250 m. L of a 3. 0% Solution From a 7. 5% Solution? Q 1: Dilute 133 m. L of 12% solution to 400 m. L Q 2: Dilute 100 m. L of 7. 5% solution to 250 m. L. . 29

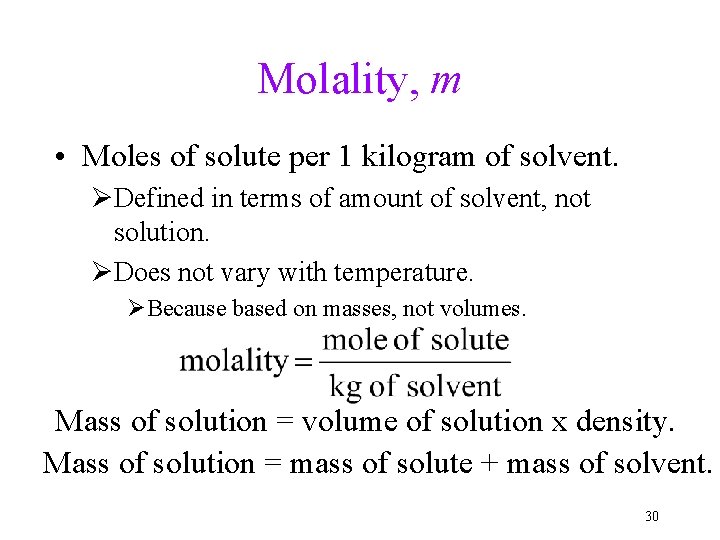
Molality, m • Moles of solute per 1 kilogram of solvent. ØDefined in terms of amount of solvent, not solution. ØDoes not vary with temperature. ØBecause based on masses, not volumes. Mass of solution = volume of solution x density. Mass of solution = mass of solute + mass of solvent. 30

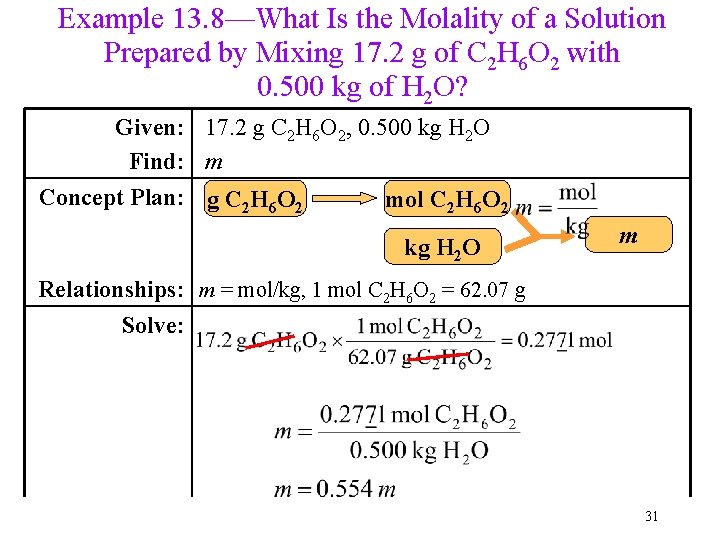
Example 13. 8—What Is the Molality of a Solution Prepared by Mixing 17. 2 g of C 2 H 6 O 2 with 0. 500 kg of H 2 O? Given: 17. 2 g C 2 H 6 O 2, 0. 500 kg H 2 O Find: m Concept Plan: g C 2 H 6 O 2 mol C 2 H 6 O 2 kg H 2 O m Relationships: m = mol/kg, 1 mol C 2 H 6 O 2 = 62. 07 g Solve: 31

Practice—What Is the Molality of a Solution that Is Made by Dissolving 3. 4 g of NH 3 (MM 17. 03) in 1500 m. L of H 2 O (d =0. 90 g/m. L). 0. 15 m 32

Colligative Properties • Colligative properties-- properties whose value depends only on the number of solute particles and not on the type. • Examples: ØVapor pressure ØFreezing point ØBoiling point ØOsmotic Pressure 33 33

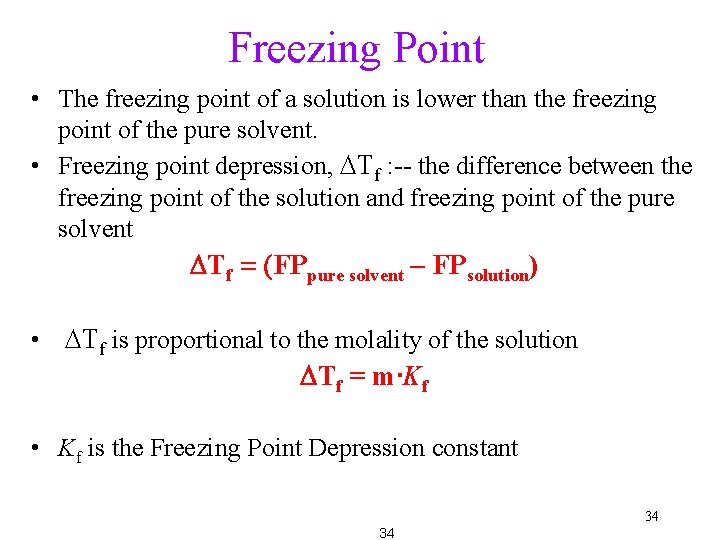
Freezing Point • The freezing point of a solution is lower than the freezing point of the pure solvent. • Freezing point depression, DTf : -- the difference between the freezing point of the solution and freezing point of the pure solvent DTf = (FPpure solvent – FPsolution) • DTf is proportional to the molality of the solution DTf = m∙Kf • Kf is the Freezing Point Depression constant 34 34

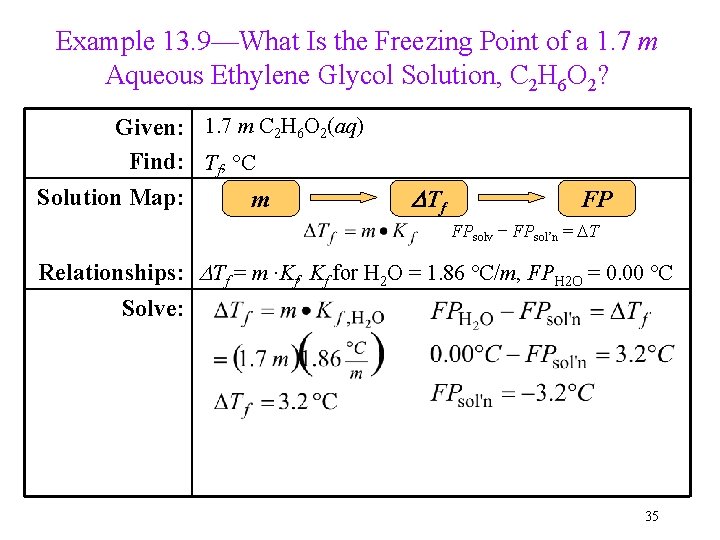
Example 13. 9—What Is the Freezing Point of a 1. 7 m Aqueous Ethylene Glycol Solution, C 2 H 6 O 2? Given: 1. 7 m C 2 H 6 O 2(aq) Find: Tf, °C Solution Map: m DTf FP FPsolv − FPsol’n = DT Relationships: DTf = m ∙Kf, Kf for H 2 O = 1. 86 °C/m, FPH 2 O = 0. 00 °C Solve: 35

Practice—What Is the Freezing Point of a Solution that Has 0. 20 moles of Sulfur Dissolved in 0. 10 kg of Cyclohexane? (FPcyclohexane = 6. 5 C, Kf = 20. 0 C/m) -33. 5 o. C 36

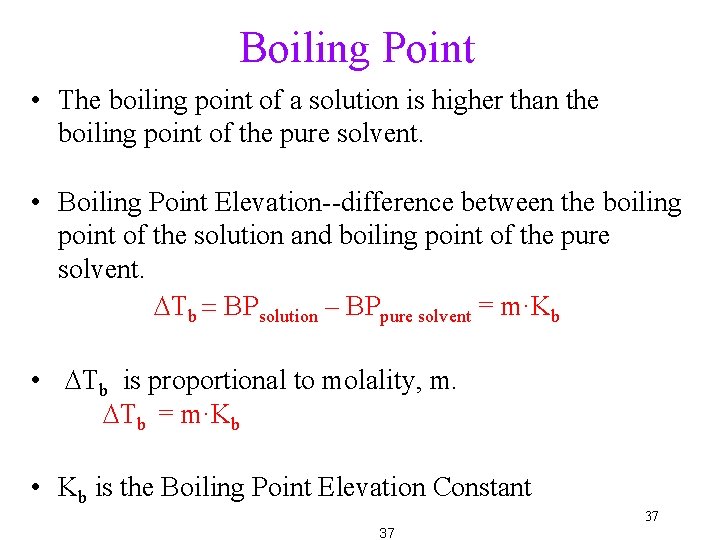
Boiling Point • The boiling point of a solution is higher than the boiling point of the pure solvent. • Boiling Point Elevation--difference between the boiling point of the solution and boiling point of the pure solvent. DTb = BPsolution – BPpure solvent = m∙Kb • DTb is proportional to molality, m. DTb = m∙Kb • Kb is the Boiling Point Elevation Constant 37 37

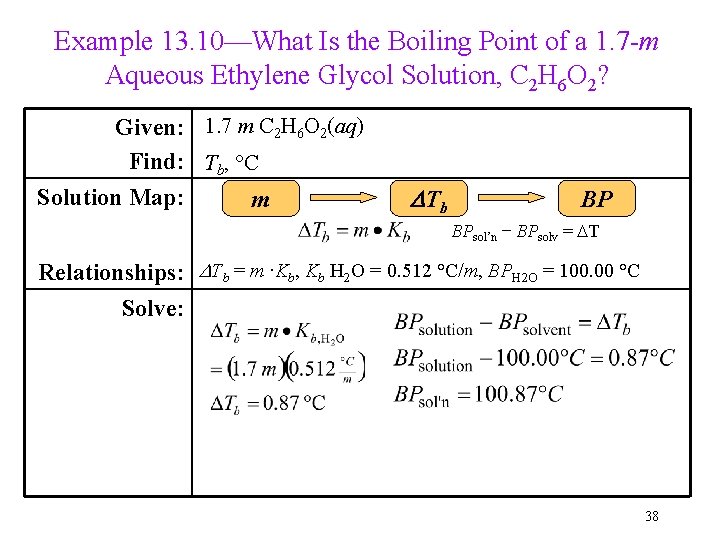
Example 13. 10—What Is the Boiling Point of a 1. 7 -m Aqueous Ethylene Glycol Solution, C 2 H 6 O 2? Given: 1. 7 m C 2 H 6 O 2(aq) Find: Tb, °C Solution Map: m DTb BP BPsol’n − BPsolv = DT Relationships: DTb = m ∙Kb, Kb H 2 O = 0. 512 °C/m, BPH 2 O = 100. 00 °C Solve: 38

Practice—What Is the Boiling Point of a Solution that Has 0. 20 moles of Sulfur Dissolved in 0. 10 kg of Cyclohexane? (BPcyclohexane = 80. 7 C, Kb= 2. 79 C/m) 86. 3 o. C 39

Osmosis • Osmosis--flow of solvent from a solution of low concentration into a solution of high concentration. • The solutions may be separated by a semipermeable membrane. • Semipermeable membrane-- allows solvent to flow through it, but not solute. 40 40

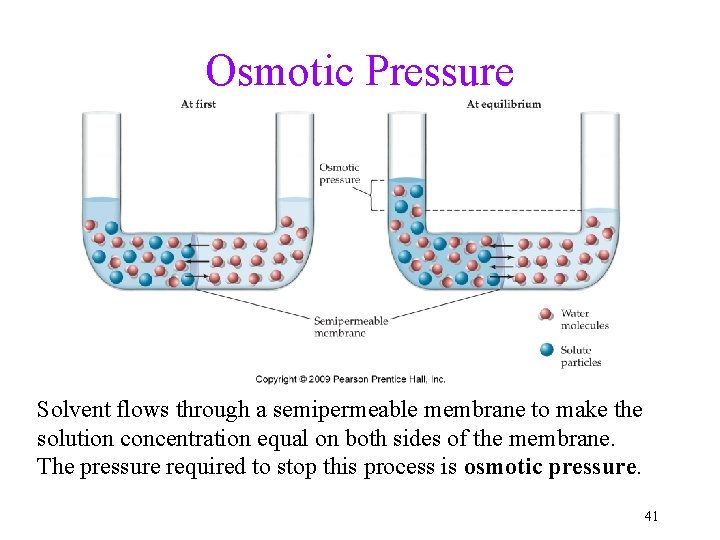
Osmotic Pressure Solvent flows through a semipermeable membrane to make the solution concentration equal on both sides of the membrane. The pressure required to stop this process is osmotic pressure. 41

Types of Osmotic Solutions • Isotonic solution: Same solute concentration relative to another solution • Hypotonic solution: Low solute concentration relative to another solution • Hypertonic solution: High solute concentration relative to another solution 42

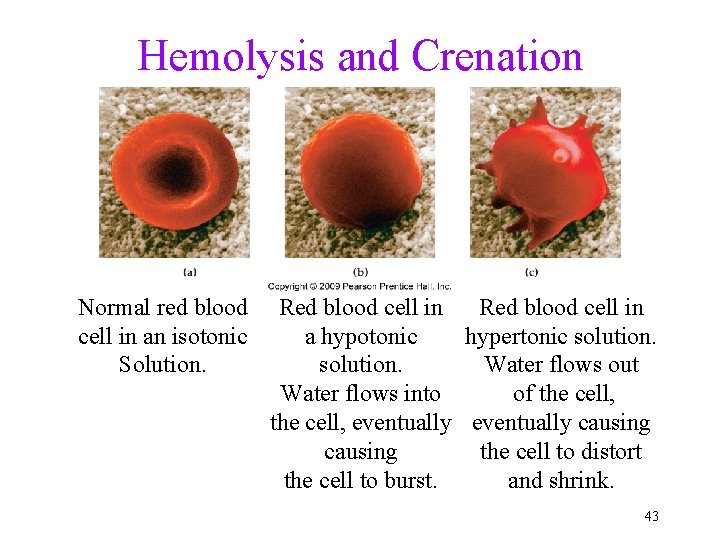
Hemolysis and Crenation Normal red blood cell in an isotonic Solution. Red blood cell in a hypotonic hypertonic solution. Water flows out Water flows into of the cell, eventually causing the cell to distort the cell to burst. and shrink. 43

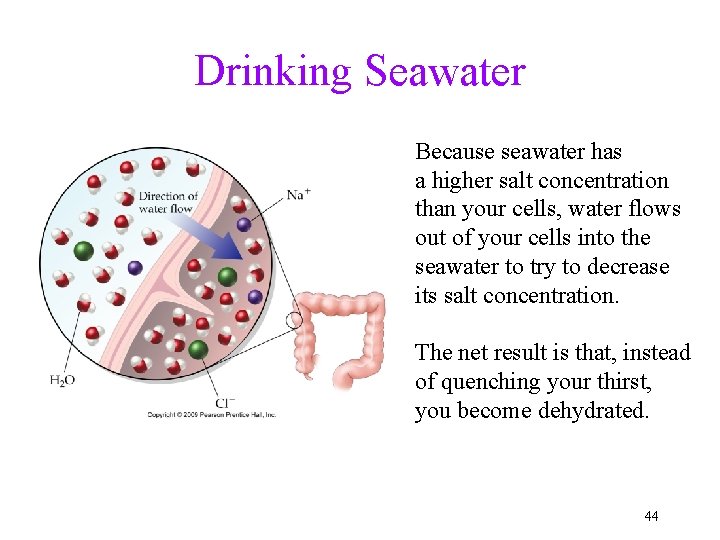
Drinking Seawater Because seawater has a higher salt concentration than your cells, water flows out of your cells into the seawater to try to decrease its salt concentration. The net result is that, instead of quenching your thirst, you become dehydrated. 44

Recommended Study Problems: Chapter 13 NB: Study problems are used to check the student’s understanding of the lecture material. Students are EXPECTED TO BE ABLE TO SOLVE ALL THE SUGGESTED STUDY PROBLEMS. If you encounter any problems, please talk to your professor or seek help at the HACC-Gettysburg learning center. Questions from text book Chapter 13 5, 6, 7, 11, 13, 17, 21, 22, 23, 24, 25, 27, 33, 37, 43, 45, 49, 51, 65, 67, 69, 73, 75, 79, 83, 85, 87, 91, 93, 99, 103, 105, 107 ANSWERS -The answers to the odd-numbered study problems are found at the back of your textbook (see Appendix III) 45