Formation of anions negative ions Cl 1 1









- Slides: 17

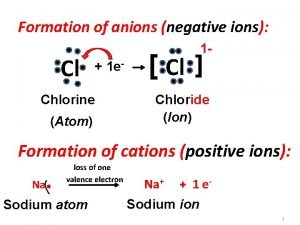
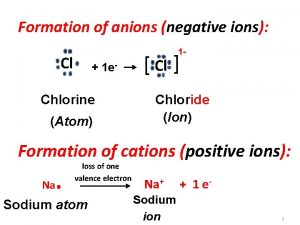
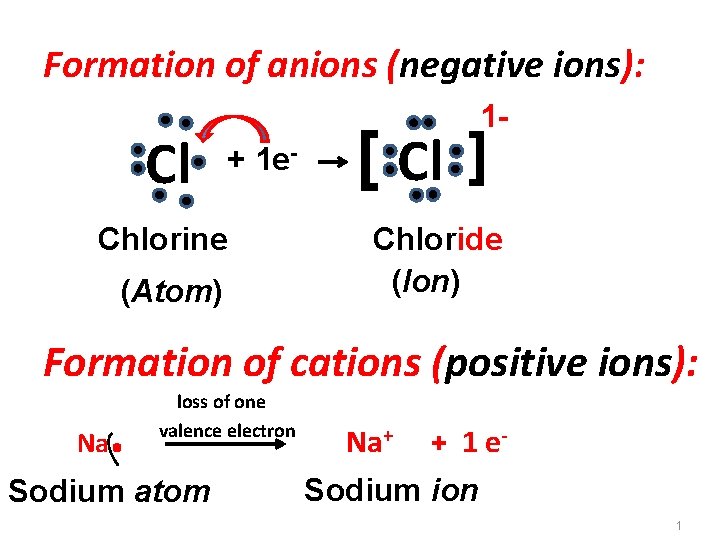
Formation of anions (negative ions): Cl 1 - + 1 e- Chlorine (Atom) [ Cl ] Chloride (Ion) Formation of cations (positive ions): Na . loss of one valence electron Sodium atom Na+ + 1 e- Sodium ion 1

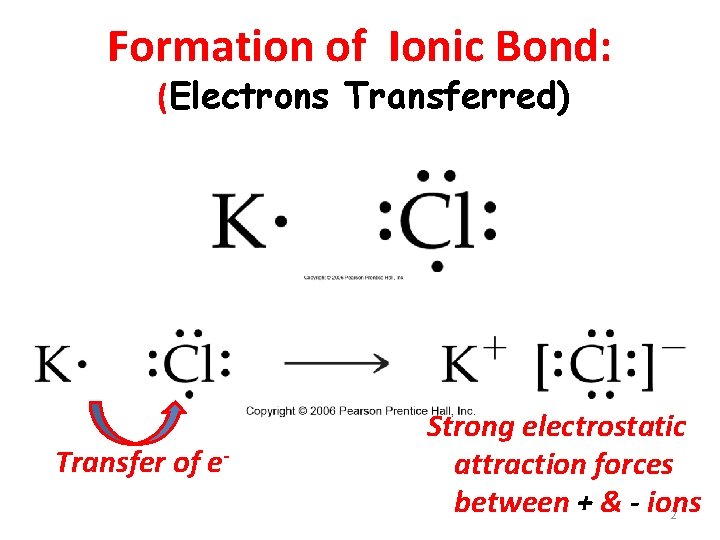
Formation of Ionic Bond: (Electrons Transferred) Transfer of e- Strong electrostatic attraction forces between + & - ions 2

IONIC BONDS: An ionic bond is formed between atoms by the transfer of one or more electrons from one atom (metal) to the other (nonmetal) The electrostatic forces than hold ions (+) & (-) together in ionic compounds are called Ionic Bond.

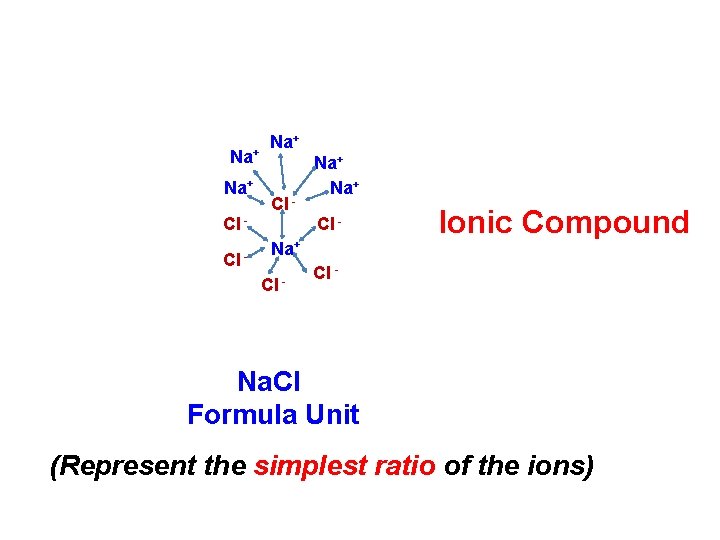
Na+ Cl Cl - Na+ Na+ Cl - Ionic Compound Cl - Na. Cl Formula Unit (Represent the simplest ratio of the ions)

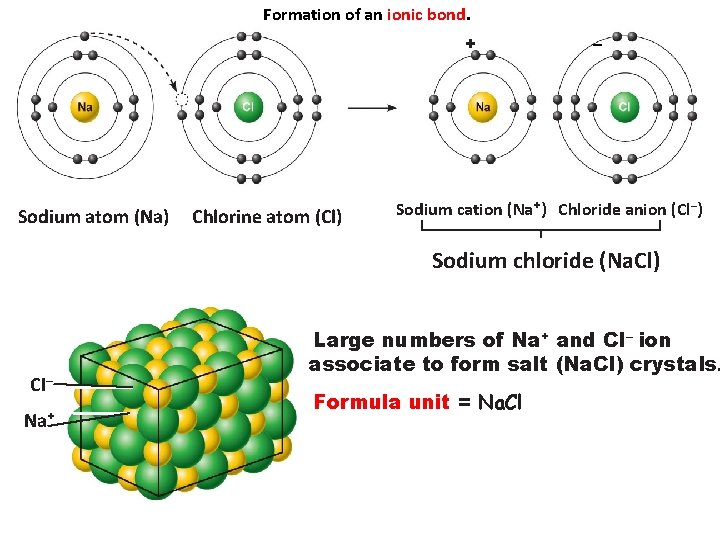
Formation of an ionic bond. + Sodium atom (Na) Chlorine atom (Cl) – Sodium cation (Na+) Chloride anion (Cl–) Sodium chloride (Na. Cl) CI– Na+ Large numbers of Na+ and Cl– ion associate to form salt (Na. Cl) crystals. Formula unit = Na. Cl

Properties of ionic compounds: 1) Strong attraction forces between positive ions and negative ions form a crystal lattice. 2) Solids at room temperature. 3) Hard, rigid, and brittle solids. 4) High melting and boiling points. 5) High solubility in water. 6) Good conductors of electricity when molten or in solution.

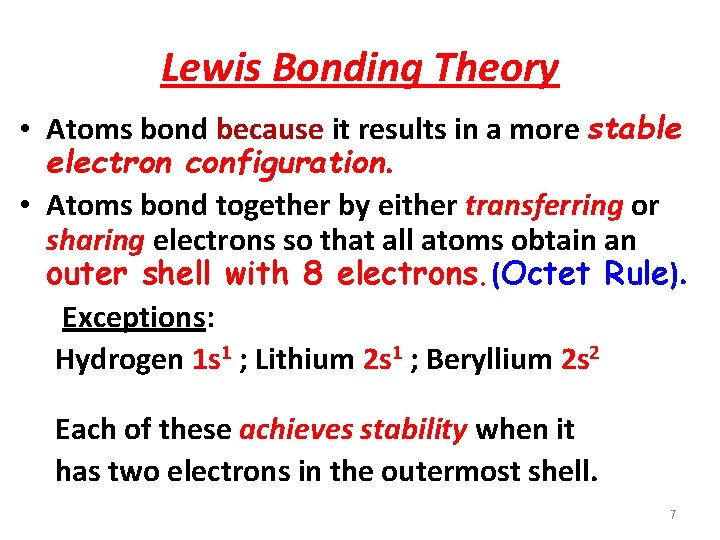
Lewis Bonding Theory • Atoms bond because it results in a more stable electron configuration. • Atoms bond together by either transferring or sharing electrons so that all atoms obtain an outer shell with 8 electrons. (Octet Rule). Exceptions: Hydrogen 1 s 1 ; Lithium 2 s 1 ; Beryllium 2 s 2 Each of these achieves stability when it has two electrons in the outermost shell. 7

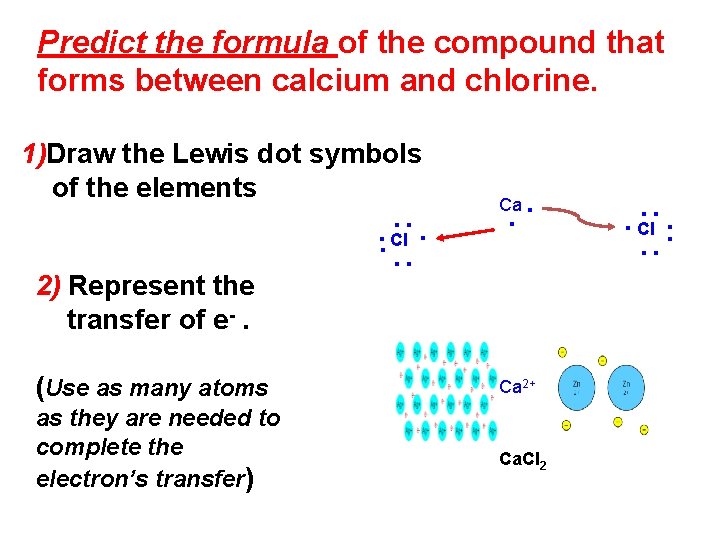
Predict the formula of the compound that forms between calcium and chlorine. ∙∙ ∙∙ ∙∙ ∙ Cl 2) Represent the transfer of e-. (Use as many atoms as they are needed to complete the electron’s transfer) ∙∙ Ca Ca 2+ Ca. Cl 2 ∙ Cl ∙∙ ∙∙ ∙∙ 1)Draw the Lewis dot symbols of the elements

Exercises: 1) An ionic bond is a. attraction of an atom for its electrons. b. attraction of atoms for electrons they share. c. a force that holds together atoms that are oppositely charged. d. the movement of electrons from one atom to another.

Exercises: 2) The formula unit of an ionic compound shows the a. total number of each kind of ion in a sample. b. simplest ratio of the ions. c. numbers of atoms within each molecule. d. number of nearest neighboring ions surrounding each kind of ion.

Exercises: 3) The overall charge of a formula unit for an ionic compound a. is always zero. c. is always positive. b. may have any value. d. is always negative. 4) How many chloride (Cl-) ions are present in a formula unit of magnesium chloride, given that the charge on a Mg ion is 2+? a. one-half b. one c. two d. four

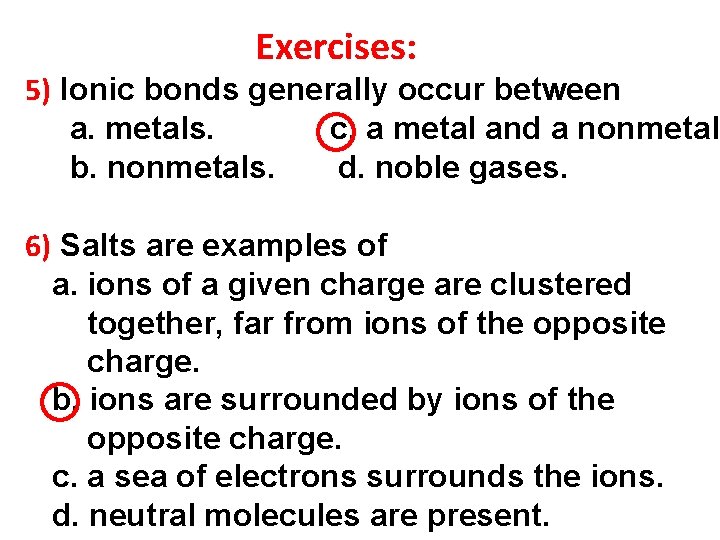
Exercises: 5) Ionic bonds generally occur between a. metals. c. a metal and a nonmetal. b. nonmetals. d. noble gases. 6) Salts are examples of a. ions of a given charge are clustered together, far from ions of the opposite charge. b. ions are surrounded by ions of the opposite charge. c. a sea of electrons surrounds the ions. d. neutral molecules are present.

Exercises: 7) In electron transfer involving a metallic atom and a nonmetallic atom during ion formation, which of the following is correct? a. The metallic atom gains electrons from the nonmetallic atom. b. The nonmetallic atom gains electrons from the metallic atom. c. Both atoms gain electrons. d. Neither atom gains electrons

Exercises: 8) Underline the word that correctly describes each property in ionic compounds: - Melting point - Boiling point - Hardness - Electrical conductivity in the solid state - Electrical conductivity in the liquid state -Electrical conductivity when dissolved in water Low Flexible High Brittle Good Poor

Metallic Bond: When metal atoms bond together their outer most energy levels overlap & the valence electrons form a “sea” of electrons which can move easily from one atom to the next.

Silver: Metallic bond: Is the strong attraction forces between the free-floating valence electrons (-) and the metallic cations (+).

Properties of Metallic Compounds: 1) Solids at room temperature. 2) Very high melting & boiling points. 3) Shiny, malleable (can be converted into sheets) and ductile (can be converted into wire). 4) Good conductors of electricity & heat. 5) Do not dissolve in water.
 Dicarbon tetraoxide
Dicarbon tetraoxide Carbon trichloride
Carbon trichloride The formation of negative ions
The formation of negative ions Formation of complex ions
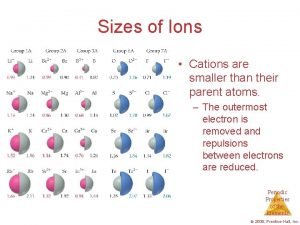
Formation of complex ions Sizes of ions
Sizes of ions Three ionic compounds
Three ionic compounds Cations and anions table
Cations and anions table What is the brown ring in the brown ring test
What is the brown ring in the brown ring test Cations and anions list
Cations and anions list Cathode vs anode equation
Cathode vs anode equation Classification of anions
Classification of anions Reactivity series of anions
Reactivity series of anions Hf acid or base
Hf acid or base Cations and anions quiz
Cations and anions quiz Unmeasured anions
Unmeasured anions Major physiological anions
Major physiological anions Positive negative attract each other
Positive negative attract each other Formation initiale vs formation continue
Formation initiale vs formation continue