Volumetric Analysis Oxidation Reduction Chapter 15 Potassium Permanganate










- Slides: 17

Volumetric Analysis Oxidation- Reduction Chapter 15

Potassium Permanganate KMn. O 4 • Potassium Permanganate is an oxidising agent. • It is a purple coloured solid. • It is not a primary standard as it decomposes in the presence of sunlight. • It acts as an oxidising agent by gaining 5 electrons in acidic solution. Mn. O 4 - + 8 H+ + 5 e- Mn 2+ + 4 H 2 O

Ammonium Iron (II) Sulphate Primary standard for KMn. O 4 Primary standard for redox titration’s: Ammonium Iron (II) Sulphate. · Stable-does not react with the gases in the air and has good solubility. · High relative molecular mass of Ammonium Iron (II) Sulphate(392) ensures a high degree of accuracy when weighing. · When dissolving in water, sulphuric acid must be added to prevent it from reacting with the water (HYDROLYSIS) and oxygen present. Fe 2+ oxidised to Fe 3+. Solutions should be made up with Distilled water as deionised water may contain chlorine

Oxidising agent-KMn. O 4 Potassium Permanganate (KMn. O 4) is a good Oxidising Agent. It itself is reduced(gains 5 electrons). · Because KMn. O 4 is not very soluble in water a dilute solution (0. 02 mol L-1) is used · The Mn. O 4 - ion is purple and Mn 2+ is colourless, so no indicator is needed. The endpoint is a permanent pale pink colour. · The meniscus is read from the top. · The H+ ions are provided by H 2 SO 4. o HCl would be oxidised o HNO 3 is an oxidising agent. · If sufficient H 2 SO 4 is not added the Mn. O 4 - is reduced to Mn. O 2 which is a brown solid. More H 2 SO 4 should be added.

Experiments 1. (Expt 4. 5) To prepare a standard solution of Ammonium Iron(II) Sulphate. And to use this solution to standardise a solution of KMn. O 4 by titration. (Page 193 of Book)

Questions and Answers Solutions to student question 1. Why is ammonium iron(II) sulfate suitable as a primary standard? Because it is stable and available in a highly pure form. 2. Why is sulfuric acid added to the iron(II) solution prior to titration? Could hydrochloric acid or nitric acid be used instead of sulfuric acid? Explain. Acidic conditions are necessary, because in neutral or alkaline conditions Mn+7 is reduced only as far as Mn+4. Hydrochloric acid is not suitable as it would react with the KMn. O 4, and chlorine gas would be evolved. Nitric acid is not suitable as it is itself a very powerful oxidising agent - the NO 3 - ion is readily reduced. 3. In preparing for the titration, explain (a) why the pipette and burette were rinsed with deionised water followed by a little of the solutions they were to contain, (b) why the conical flask was rinsed with deionised water only. (a) Deionised water washes out any residual solutions in the burette and pipette respectively. The second step was taken to remove any residual water, and so avoid dilution of the solutions when they are added to the burette and pipette respectively. (b) Deionised water washes out any residual solution in the conical flask. If it were then washed out with the solution it was to contain, traces of it would remain, and there would not be a precisely known amount of the solution in the flask.

4. During the titration the sides of the conical flask were washed down with deionised water from a wash bottle. Explain why this procedure is necessary and why it can be carried out without affecting the result of the titration. The washing process was carried out to ensure that all of the manganate(VII) solution added from the burette reacted with the iron(II) solution. It did not affect the result of the titration because only deionised water was added – no extra reactants were introduced into the flask. 5. One of the products of this reaction acts as a catalyst for the reaction. Which product is this? How could you demonstrate what substance is acting as the catalyst? The reaction is catalysed by Mn 2+ ions. This can be shown by taking a clean conical flask, pipetting the Fe 2+ solution into it, acidifying it and then before starting to titrate adding some Mn. SO 4 solution (a convenient source of Mn 2+). Now the first droplet of Mn. O 4 - added decolourises immediately as there is Mn 2+ in place to act as catalyst. 6. Why was sulfuric acid added in making up the ammonium iron(II) sulfate solution? Iron(II) is very susceptible to air oxidation, forming iron(III), under neutral or alkaline conditions but this oxidation is inhibited in the presence of acids. The ammonium iron(II) sulfate solution is made up in dilute acid solution to make it stable towards air oxidation.

Iron in Iron Tablet 2. (Expt 4. 6) Using the standardised KMn. O 4 to determine the amount of Iron in an Iron tablet. (Page 197 of Book). (Q 1. Leaving Cert 2003)

Questions and Answers Solutions to student questions 1. In this experiment why is dilute sulfuric acid used rather than deionised water to dissolve the iron tablets? If deionised water were used, the Fe 2+ in the tablets would be almost immediately oxidised to Fe 3+. The sulfuric acid prevents this occurring. 2. Why are burette readings taken from the top of the meniscus? Because the very dark colour of the manganate(VII) solution makes the meniscus difficult to see. 3. How is the end-point of the titration detected? When the first permanent pale pink colour forms in the solution in the conical flask. 4. Why is a rough titration carried out? To determine the approximate end-point. This can then be used to get accurate results in the subsequent titrations.

5. Why is more than one titration carried out subsequently? To reduce experimental error, by getting the mean of the accurate titres. 6. Prior to the titration, what steps are taken to minimise error? All glassware is washed with deionised water. The burette and pipette respectively are rinsed with the solution they are to contain. The tap of the burette is opened briefly to fill the part of the burette below the tap. 7. If a brown precipitate appears during the titration, what does this indicate, and how can it be remedied? Mn(IV) is formed, because of incomplete reduction of the Mn(VII). This should only happen if there is insufficient sulfuric acid in the conical flask. The remedy is to add more dilute sulfuric acid to the flask, or, preferably, to repeat the experiment with sufficient acid present in the flask.

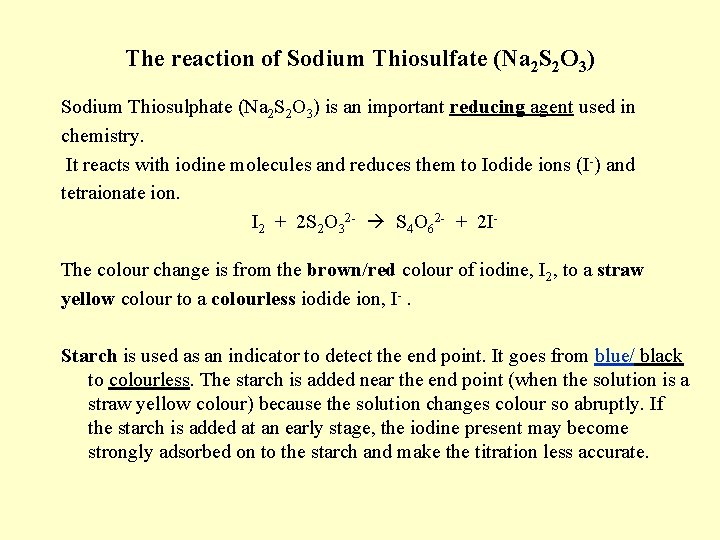
The reaction of Sodium Thiosulfate (Na 2 S 2 O 3) Sodium Thiosulphate (Na 2 S 2 O 3) is an important reducing agent used in chemistry. It reacts with iodine molecules and reduces them to Iodide ions (I-) and tetraionate ion. I 2 + 2 S 2 O 32 - S 4 O 62 - + 2 IThe colour change is from the brown/red colour of iodine, I 2, to a straw yellow colour to a colourless iodide ion, I-. Starch is used as an indicator to detect the end point. It goes from blue/ black to colourless. The starch is added near the end point (when the solution is a straw yellow colour) because the solution changes colour so abruptly. If the starch is added at an early stage, the iodine present may become strongly adsorbed on to the starch and make the titration less accurate.

Standardising Sodium Thiosulfate Sodium thiosulphate is not a primary standard. It is standardised by titration it against acidified potassium permanganate with an excess of acidified potassium iodide. When potassium permanganate reacts with potassium iodide, the potassium iodide is oxidised to free iodine (I 2). 2 Mn. O 4 - + 10 I- + 16 H+ ¾¾¾® 2 Mn 2+ + 5 I 2 + 8 H 2 O I 2 + 2 S 2 O 32 - ¾¾¾® S 4 O 62 - + 2 IThe free iodine can then be titrated against the unknown thiosulphate solution using starch as an indicator. The overall reactions are: 2 mol KMn. O 4 = 5 mol I 2 = 10 mol Na 2 S 2 O 3 The iodine cannot be used as a primary standard because it does not dissolve readily in water and iodine sublimes.

To determine the percentage (w/v) of sodium hypochlorite in bleach Many commercial bleaches are simply solutions of hypochlorite salts such as sodium hypochlorite (Na. OCl) or calcium hypochlorite (Ca(OCl)2). Hypochlorite ion reacts with excess iodide ion in the presence of acid to generate an iodine solution: Cl. O- + 2 I- + 2 H+ Cl- + I 2 + H 2 O The liberated iodine solution can be titrated against sodium thiosulfate solution using a freshly prepared starch solution as indicator. The titration reaction may be represented by the equation: I 2 + 2 S 2 O 322 I- + S 4 O 62 Starch indicator is added during the titration when the colour of the solution in the conical flask fades to a pale yellow colour. The solution becomes blue black, and the titration is continued until it goes colourless.

Experiments 1. (Expt. 4. 7) To prepare a solution of sodium thiosulfate and standardise it by titration against a solution of iodine. (S ee page 202 book)

Questions and Answers Solutions to student questions 1. Why is hydrated sodium thiosulfate not suitable as a primary standard? It loses water of crystallisation readily, and it is not stable. 2. Why are iodine solutions made up using potassium iodide solution? Iodine is sparingly soluble in water. However it reacts with iodide forming I 3 - ions, which are very soluble. In this way the iodine is kept in solution. 3. Why does starch solution have to be freshly prepared? It deteriorates quickly on standing. 4. Which of the three pieces of titration apparatus, the pipette, the burette or the conical flask, should not be rinsed with the solution it is to contain? Give a reason for your answer. The conical flask should not be rinsed with the solution it is to contain. If it were washed out with the solution it was to contain, then traces of the solution would remain, and there would not be a precisely known amount of the solution in the flask. 5. Why is starch indicator added close to the end-point? To give a sharp end-point, while avoiding the formation of excess starch-iodine complex, which would be difficult to decompose. 6. What happens at the end-point? The colour changes from blue-black to colourless.

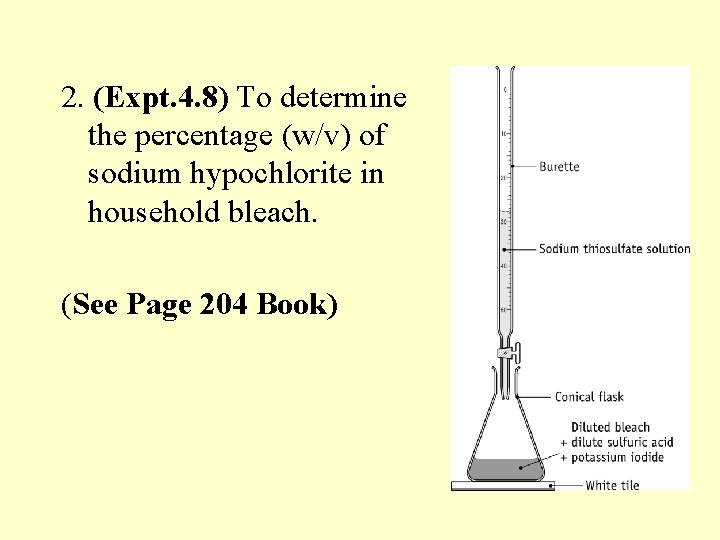
2. (Expt. 4. 8) To determine the percentage (w/v) of sodium hypochlorite in household bleach. (See Page 204 Book)

Questions and Answers Solutions to student questions 1. Give one reason why, in making up the solution of diluted bleach, a volumetric flask is preferable to a graduated cylinder. A volumetric flask is quite an accurate measuring vessel, whereas a graduated cylinder is not. 2. A burette, a pipette and a conical flask were used in the titration. State the correct washing procedures for each of these items before starting the titration. Rinse the burette, pipette and conical flask respectively with deionised water. Rinse the burette with sodium thiosulfate solution, and rinse the pipette with diluted bleach solution. 3. Why could you not use hydrochloric acid when acidifying the bleach? Hydrochloric acid is not suitable, as it will react with hypochlorite to liberate chlorine gas.