SOLUTIONS Solution a homogeneous mixture of two or










- Slides: 11

SOLUTIONS

Solution a homogeneous mixture of two or more substances Homogeneous - having similarity in structure mixtures-two or more substances physically mixed together but not chemically combined

TYPES AQUEOUS solutions a solution in water TINCTURE a solution in alcohol

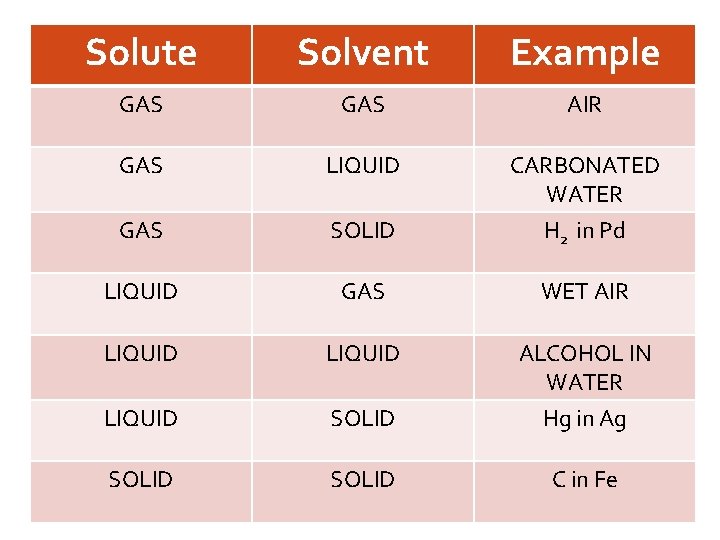
COMPONENTS Solute the substance being dissolved present in lesser quantity. Solvent the substance where the solute is dissoved in; medium present in greater quantity

Solute Solvent Example GAS AIR GAS LIQUID CARBONATED WATER Solute the substance SOLIDbeing dissolved H in Pd GAS 2 present in lesser quantity. LIQUID GAS Solvent LIQUID the LIQUID WET AIR ALCOHOL IN WATER substance where the solute SOLID Hg in Ag is dissoved in; medium SOLID present in greater quantity C in Fe

REVERSIBLE INTERACTIONS Solvation the process of surrounding a solute particle with solvent particles. Hydration is solvation in water Crystallization

Solvation Step 1. Solute particles separate from each other. This step involves overcoming intermolecular attractions, so it is endothermic: Solute (aggregated) + heat solute (separated)

Solvation Step 2. Solvent particles separate from each other. This step also involves overcoming attractions, so it is endothermic, too: Solvent (aggregated) + heat solvent (separated)

Solvation Step 3. Solute and solvent particles mix. The particles attract each other, so this step is exothermic: Solute (separated) + solvent (separated) solution + heat

Solubility The solubility of a substance is the amount of that substance that can be dissolved in a given quantity of solvent. Miscible – can mix in any proportions e. g. (liquid-liquid solns)

Saturated solution the maximum amount of solute is dissolved in the solvent at a given temperature Unsaturated the amount of solute dissolved in the solvent at a given temperature is less than maximum Supersaturated the amount of solute dissolved in the solvent at a given temperature is more than maximum