Matter and Change n Chemistry u Study and

















- Slides: 34

Matter and Change

n Chemistry: u Study and investigation of the structure and properties of matter n Chemical u all n types of matter Branches of Chemistry: u page 4

There are only two things in our universe: n Matter and Energy

Matter n n anything that occupies space and has mass anything that exhibits inertia (resistance to changes in motion (speed and direction)

Matter Defined n n Anything that takes up space (volume) and has mass Volume is the space an object occupies Mass is the amount of matter in an object Inertia can also be used to define matter – resistance to change in motion; directly proportional to amount of matter

Properties of Matter n n movie n Matter can be defined based on its PROPERTIES Properties are characteristics or behaviors of matter Properties are classified as Physical u Chemical u

Physical Properties of Matter n n n Can be measured or observed without changing the material’s composition Examples include density, color, odor, hardness, taste, melting point, and boiling point Physical properties can be further classified as INTENSIVE or EXTENSIVE

Extensive Physical Properties n n Extensive properties are dependent upon the amount of material present Examples include mass, length, volume and solubility

Intensive Physical Properties n n Intensive physical properties DO NOT depend on the amount of material present Examples include density, color, state, melting point, and boiling point

Examples of Physical Properties: n n n n n Malleability Ductility Luster Odor Hardness Refractive Index Crystalline shape Specific heat capacity Conductivity – electrical or heat

Chemical Properties of Matter n n n Properties which describe the ability of a substance to combine or change into one or more substances during a chemical reaction Describes what a material CAN DO if the conditions are favorable for a reaction to occur Examples include the ability of iron to rust, the ability of silver to tarnish, the ability of gasoline to burn and give off heat/energy

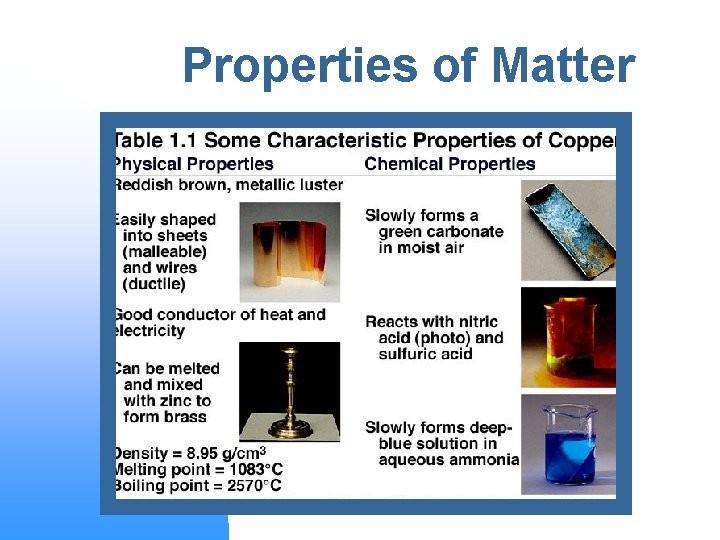
Properties of Matter

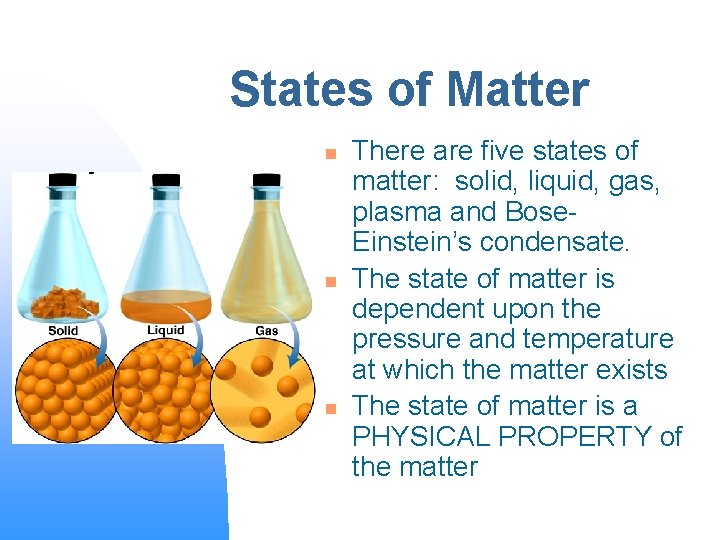
States of Matter n n n There are five states of matter: solid, liquid, gas, plasma and Bose. Einstein’s condensate. The state of matter is dependent upon the pressure and temperature at which the matter exists The state of matter is a PHYSICAL PROPERTY of the matter

Solids n n Have a definite shape and volume Particles are packed close together and vibrate weakly about fixed positions Not compressible Particles have low kinetic energy

Liquids n n Have a definite volume, no definite shape and can flow Particles can move and slide Particles are very close together so liquids are practically noncompressible Particles have more kinetic energy that solids

Gases n n n No definite shape No definite volume Particles move rapidly in all directions; low attractive forces between particles Higher kinetic energy than solid or liquid Highly compressible

Plasma n n Made up of ionized gas particles Most abundant state of matter in the universe Occurs in stars, lightning bolts, fluorescent lights Typically occur at temperatures over 5000 degrees C. at normal pressures

Bose - Einstein’s Condensate n Confirmed in 1995 u n n n (2001 Nobel prize – MIT researcher) Individual atoms meld into a super atom Occurs at a few billionths of a degree above 0 K Important in superconductivity research

Physical Changes in Matter n n n Changes that DO NOT produce new substances Examples include tearing a sheet of paper, bending a piece of metal, grinding a solid ALL PHASE CHANGES ARE PHYSICAL CHANGES Boiling u Freezing u Condensing u

Chemical Changes in Matter Changes in matter which n n produce new substances with new and different properties The chemical composition of the products is different from the chemical composition of the reactants Examples include iron rusting, wood burning, digestion of food Law of Conservation of Mass applies

Signs of chemical change: n n n Light Heat Gas Release Precipitation Electricity

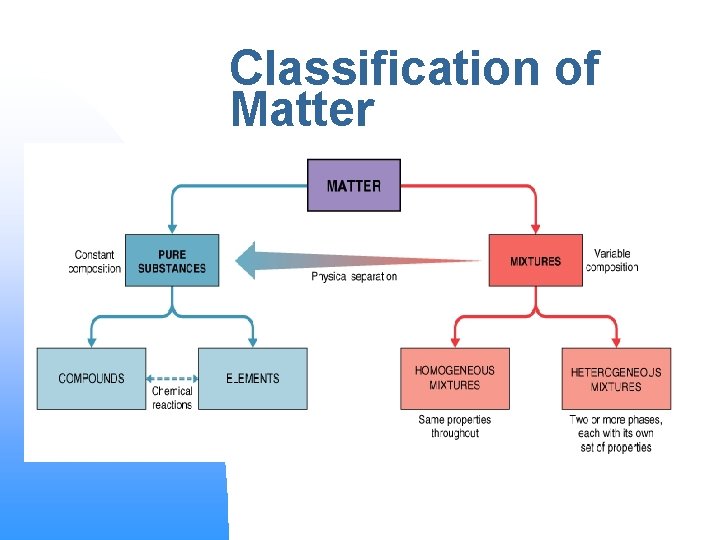
Classification of Matter

Pure Substances n n Made up of only one kind of atom or molecule Have uniform composition u n n Homogeneous Elements and Compounds Organic or Inorganic u Organic – Carbon compds.

Pure Substances n n Elements: aluminum, gold, carbon Compounds: salt, baking soda, water

Elements n n n Simplest type of matter made of one type of atoms 115 known (about 90 naturally occurring) Elements arranged according to increasing atomic mass and recurring properties in PERIODIC TABLE OF THE ELEMENTS Periodic table created by Mendeleev, refined by Mosely and Seaborg Each element has unique one or two letter symbol

Compounds n n Chemical combinations of two or more elements Most familiar substances are compounds (10 million known) Law of Definite Composition applies to compounds (formulas) Law of Multiple Proportions = whole number ratios

Mixtures: n n n 2 or more kinds of matter Composition is variable Each component retains its own properties

Heterogeneous Mixtures n n Physically distinct parts (phases) with different properties separated by interfaces Composition is NOT uniform May form layers Examples include blood, whole milk, granite, and chocolate chip cookies

Homogeneous Mixtures n n n Uniform composition Contains only 1 phase Liquid homogeneous mixtures are also called SOLUTIONS Solid homogeneous mixtures of metals are ALLOYS Examples include air, vinegar, salt water, and Sprite

Separation of Matter n n n Elements – cannot be broken down Compounds – can be broken down by chemical methods into simpler compounds and/or elements Mixtures – can be separated by physical means; method of separation depends on the properties of the matter

Separation Techniques n n n n Evaporation Distillation Chromatograph y Density Centrifuge Filtration Fractional crystallization

Nuclear Changes: n n A new substance is formed by changing the identity of the atom Protons and neutrons are changed in the nucleus Mass may be converted to energy or vice versa Examples: u Fission u Fusion u Radioactive Decay

Energy changes: n Chemical potential energy: u Energy stored in a substance because of its composition u Converted to other forms of energy in a chemical change

Energy may be transformed from one type to another: n n n Electric Mechanical Chemical Light Sound Heat