Matter Properties Change Chapter 3 Matter l Matter























- Slides: 31

Matter: Properties & Change Chapter 3

Matter l Matter – anything that has mass and takes up space l l Everything around us Chemistry – the study of matter and the changes it undergoes

Four States of Matter l Solids particles vibrate but can’t move around l fixed shape l fixed volume l incompressible l

Four States of Matter l Liquids particles can move around but are still close together l variable shape l fixed volume l l Virtually incompressible

Four States of Matter l Gases particles can separate and move throughout container l variable shape l variable volume l Easily compressed l Vapor = gaseous state of a substance that is a liquid or solid at room temperature l

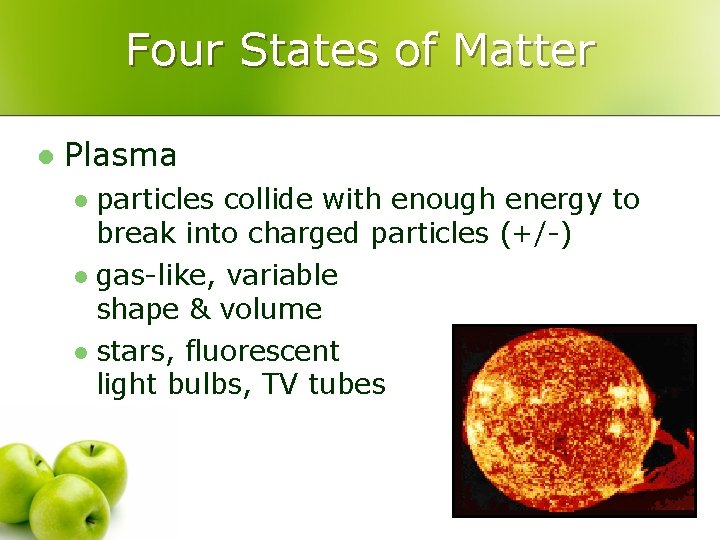
Four States of Matter l Plasma particles collide with enough energy to break into charged particles (+/-) l gas-like, variable shape & volume l stars, fluorescent light bulbs, TV tubes l

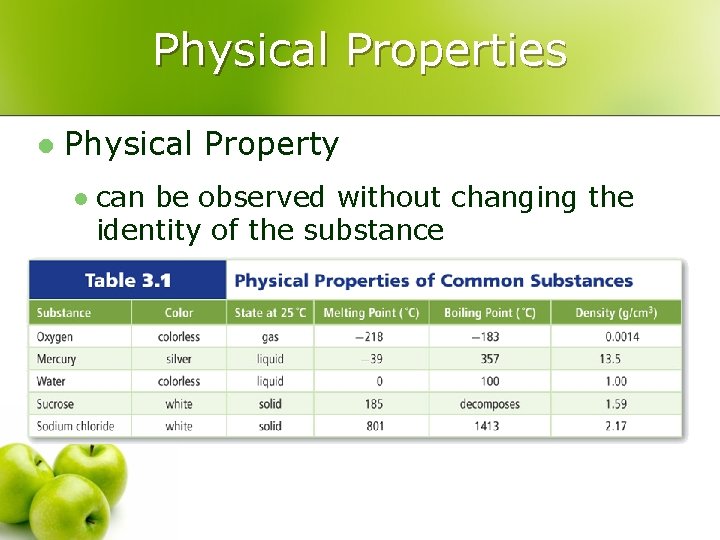
Physical Properties l Physical Property l can be observed without changing the identity of the substance

Physical Properties l Physical properties can be described as one of 2 types: l Extensive Property l l depends on the amount of matter present (example: length) Intensive Property l depends on the identity of substance, not the amount (example: scent)

Extensive vs. Intensive l Examples: l boiling point l volume l mass l density l conductivity

Density – a physical property l l Derived units = Combination of base units Volume (m 3 or cm 3 or m. L) l l length Or measured using a graduated cylinder 1 cm 3 = 1 m. L 1 dm 3 = 1 L Ø Density (kg/m 3 or g/cm 3 or g/m. L) w mass per volume M D= V

Density l An object has a volume of 825 cm 3 and a density of 13. 6 g/cm 3. Find its mass. GIVEN: V= D= M=? WORK:

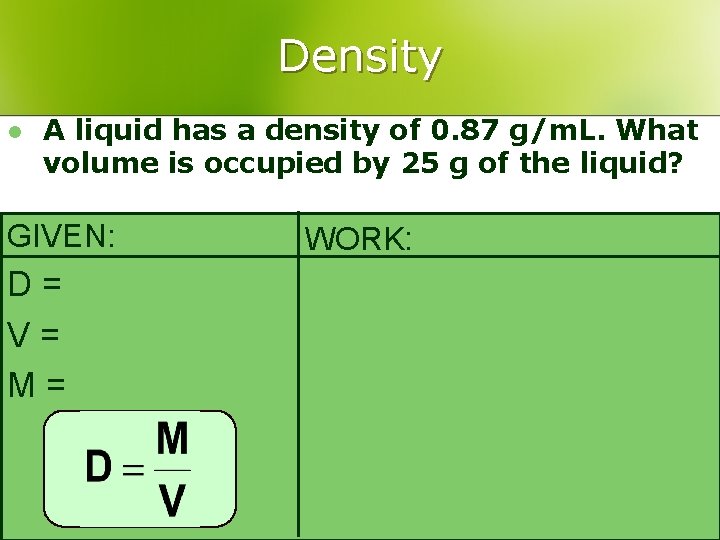
Density l A liquid has a density of 0. 87 g/m. L. What volume is occupied by 25 g of the liquid? GIVEN: D= V= M= WORK:

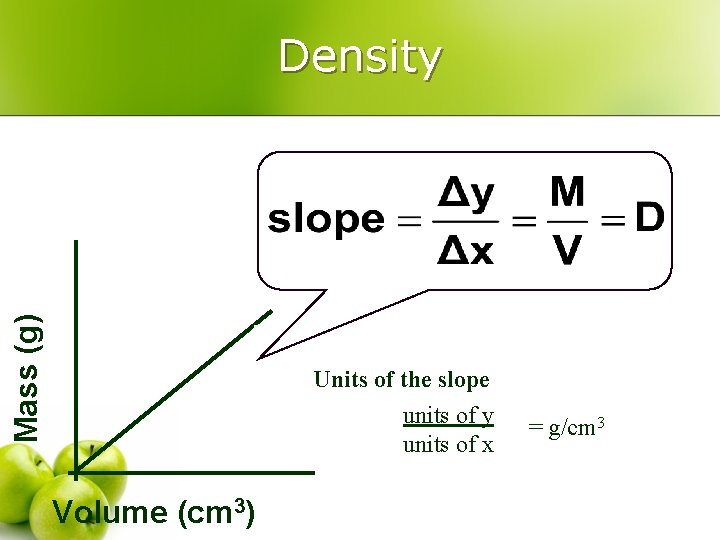
Mass (g) Density Units of the slope units of y units of x Volume (cm 3) = g/cm 3

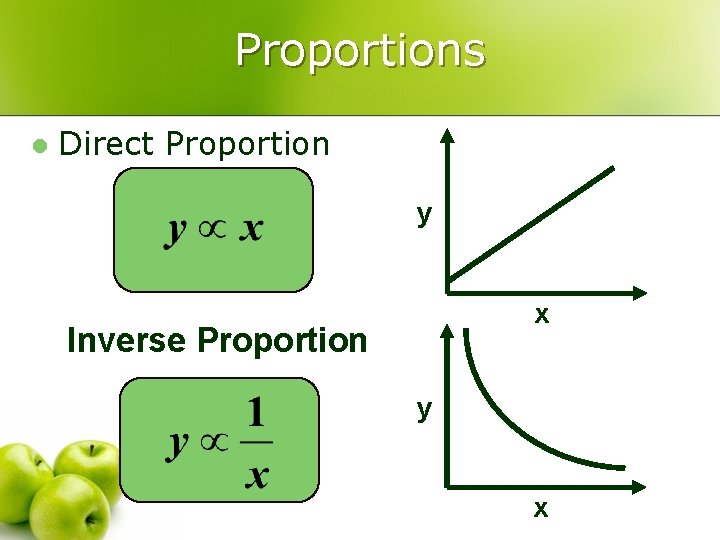
Proportions l Direct Proportion y x Ø Inverse Proportion y x

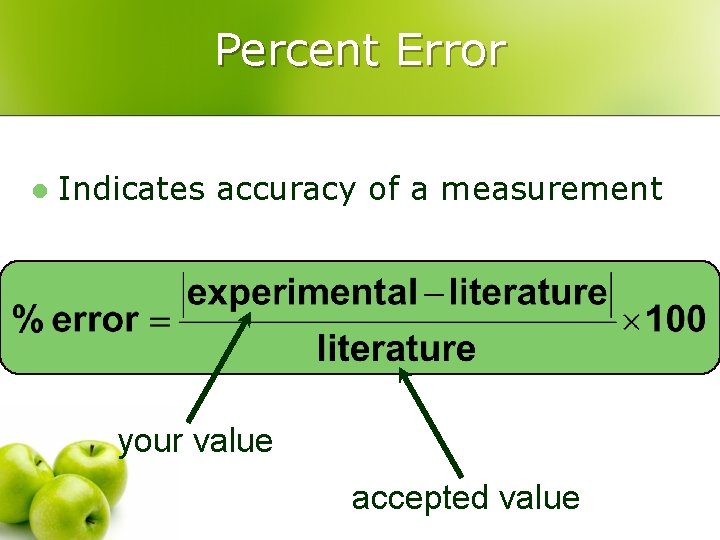
Percent Error l Indicates accuracy of a measurement your value accepted value

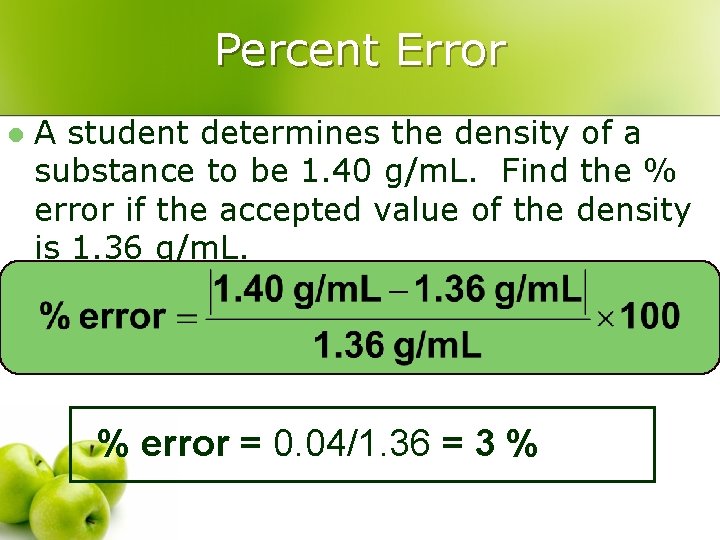
Percent Error l A student determines the density of a substance to be 1. 40 g/m. L. Find the % error if the accepted value of the density is 1. 36 g/m. L. % error = 0. 04/1. 36 = 3 %

Chemical Properties l Chemical Property l describes the ability of a substance to undergo changes in identity

Physical vs. Chemical Properties l Examples: l melting point l flammable l density l magnetic l tarnishes in air

Physical Changes l l Physical Change l changes the form of a substance without changing its identity l properties remain the same Examples: change in shape or size, dissolving, change in color by dying, all phase changes,

Phase Changes – Physical l Evaporation = l Condensation = l Melting = l Freezing = l Sublimation =

Chemical Changes l Process that involves one or more substances changing into a new substance Commonly referred to as a chemical reaction l New substances have different compositions and properties from original substances l

Chemical Changes l Signs of a Chemical Change l change in color or odor (not by dying) l formation of a gas (bubbling) l formation of a precipitate (solid) l change in light or heat

Physical vs. Chemical Changes l Examples: l rusting iron l dissolving in water l burning a log l melting ice l grinding spices

What Type of Change?

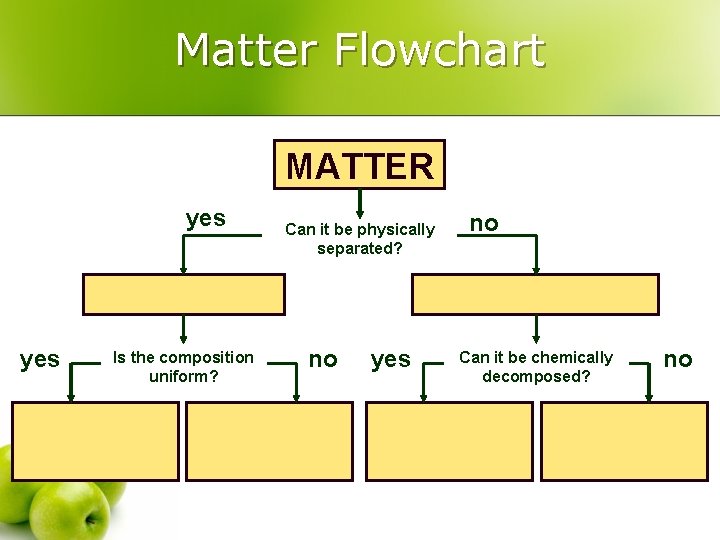
Matter Flowchart MATTER yes Is the composition uniform? Can it be physically separated? no yes no Can it be chemically decomposed? no

Pure Substances l Element composed of identical atoms l EX: copper wire, aluminum foil l

Pure Substances l Compound l composed of 2 or more elements in a fixed ratio l properties differ from those of individual elements l EX: table salt (Na. Cl)

Mixtures l Variable combination of 2 or more pure substances. Heterogeneous Homogeneous

Mixtures l Solution homogeneous l very small particles don’t settle l EX: rubbing alcohol l

Mixtures l Heterogeneous medium-sized to large-sized particles l particles may or may not settle l EX: milk, freshsqueezed lemonade l

Mixtures l Examples: l tea l muddy water l fog l saltwater l Italian salad dressing