Matter and Change What is Matter Matter is
























- Slides: 38

Matter and Change

What is Matter? Matter is anything that takes up space and has mass. Mass is the amount of matter in an object.

Physical Properties A property that can be observed and measured without changing the substance.

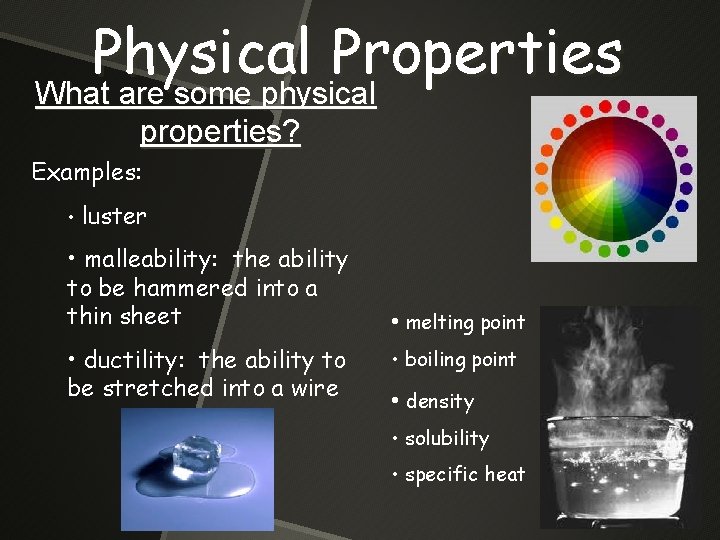
Physical Properties What are some physical properties? Examples: • luster • malleability: the ability to be hammered into a thin sheet • ductility: the ability to be stretched into a wire • melting point • boiling point • density • solubility • specific heat

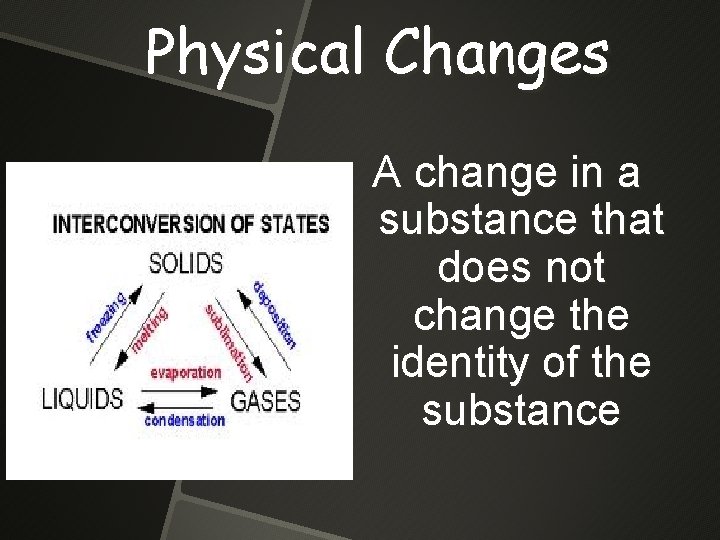
Physical Changes A change in a substance that does not change the identity of the substance

Physical Changes

Physical Changes Some physical changes would be boiling of a liquid melting of a solid dissolving a solid in a liquid to give a homogeneous mixture — a SOLUTION.

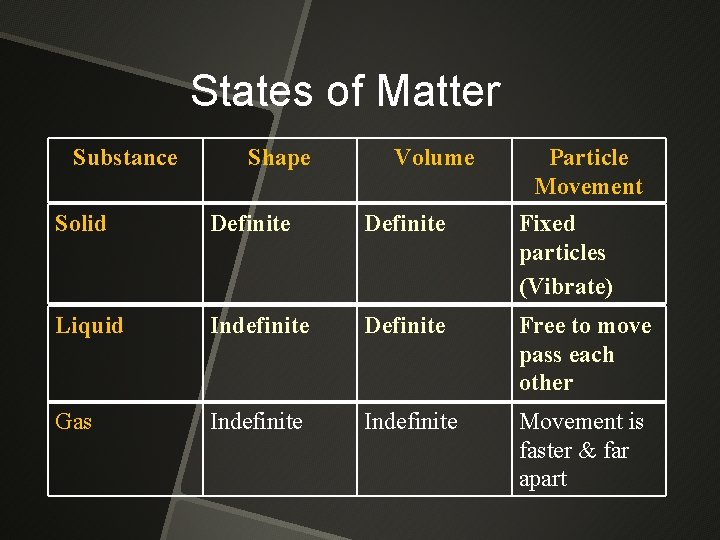
States of Matter Substance Shape Volume Particle Movement Solid Definite Fixed particles (Vibrate) Liquid Indefinite Definite Free to move pass each other Gas Indefinite Movement is faster & far apart

Chemical Properties- a property that can only be observed by changing the type of substance.

Chemical Change or Chemical Reaction Transformation of one or more substances into a different substance with different properties. You can not get the original substance back

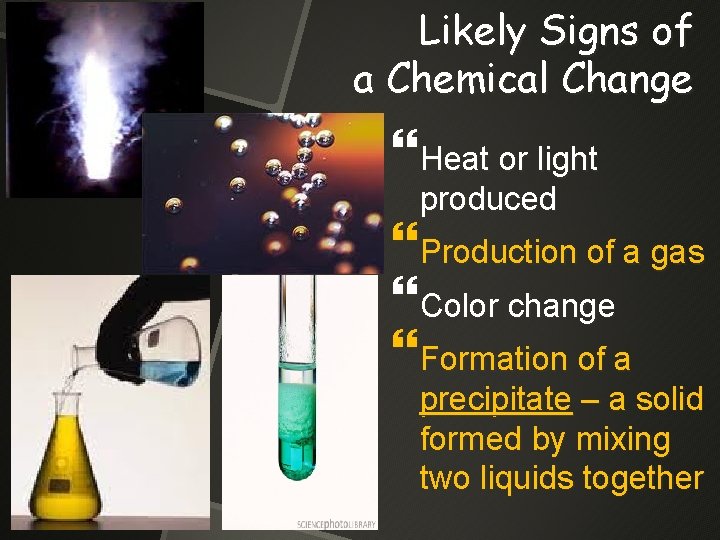
Likely Signs of a Chemical Change Heat or light produced Production of a gas Color change Formation of a precipitate – a solid formed by mixing two liquids together

Chemical Change or Chemical Reaction Examples of a chemical change: q. Iron rusts or oxidizes q. Hydrochloric acid reacts with potassium hydroxide q. Neutralization of acid with a base q. Grass growing in a lawn (Photosynthesis) q. Electrolysis of water q. Hydrolysis of water

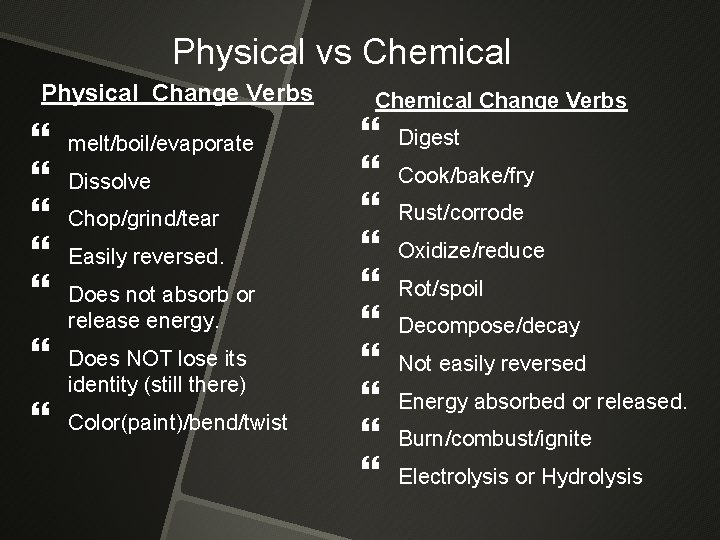
Physical vs Chemical Physical Change Verbs melt/boil/evaporate Dissolve Chop/grind/tear Easily reversed. Does not absorb or release energy. Does NOT lose its identity (still there) Color(paint)/bend/twist Chemical Change Verbs Digest Cook/bake/fry Rust/corrode Oxidize/reduce Rot/spoil Decompose/decay Not easily reversed Energy absorbed or released. Burn/combust/ignite Electrolysis or Hydrolysis

Learning Check Physical or Chemical Property? melting point flammable density magnetic tarnishes in air solubility Ø Physical Ø Chemical Ø Physical

Learning Check Physical or Chemical Change? Potassium chlorate ØChemical decomposing dissolving in water burning a log water evaporating grinding spices formation of a precipitate Boiling water ØPhysical ØChemical ØPhysical

C lass ifica ti on of Pro p e rt ie s Extensive properties Depend upon the amount of matter present Ex: mass, volume, heat energy, length

Cla s s if ic a tion of P ro p e rtie s Intensive properties Do not depend upon the amount of matter present, and remains constant Ex: density, boiling, freezing, melting points, conductivity, solubility, magnetism

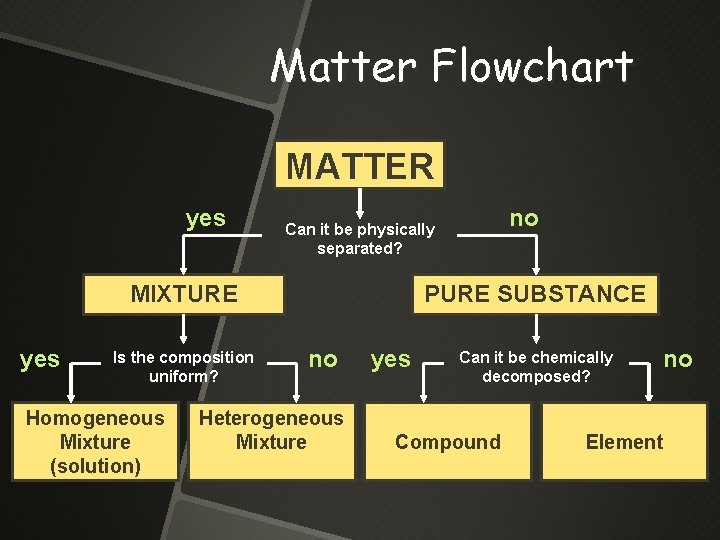
Mixtures and Pure Substances

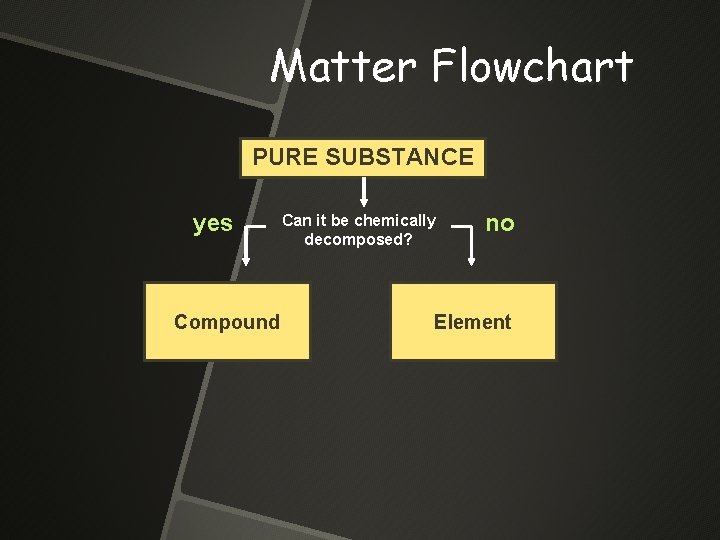
Matter Flowchart PURE SUBSTANCE yes Compound Can it be chemically decomposed? no Element

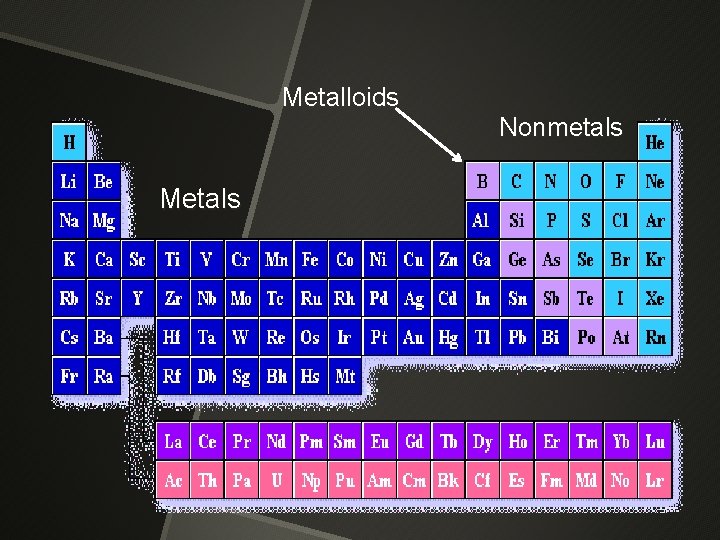
Metalloids Metals Nonmetals

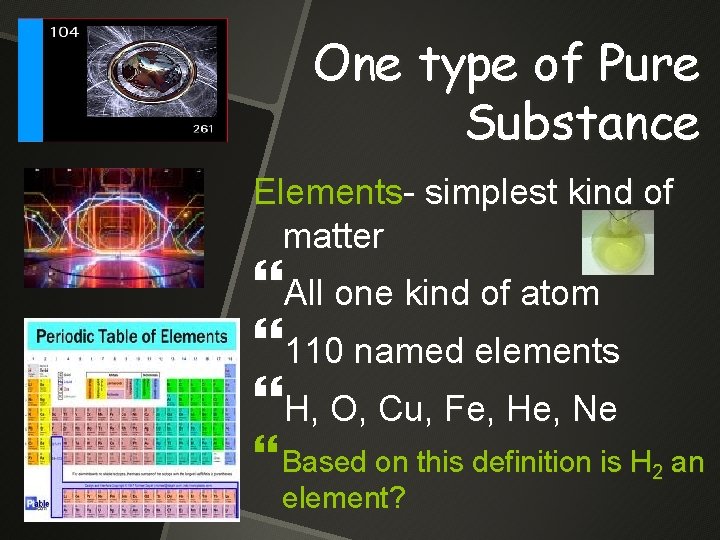
One type of Pure Substance Elements- simplest kind of matter All one kind of atom 110 named elements H, O, Cu, Fe, He, Ne Based on this definition is H 2 an element?

The other type of Pure Substance Compounds are substances that can be broken down by a chemical change Atoms of two or more different elements that have been combined in a fixed proportion. H 2 O, Na. Cl, Ca. CO 3,

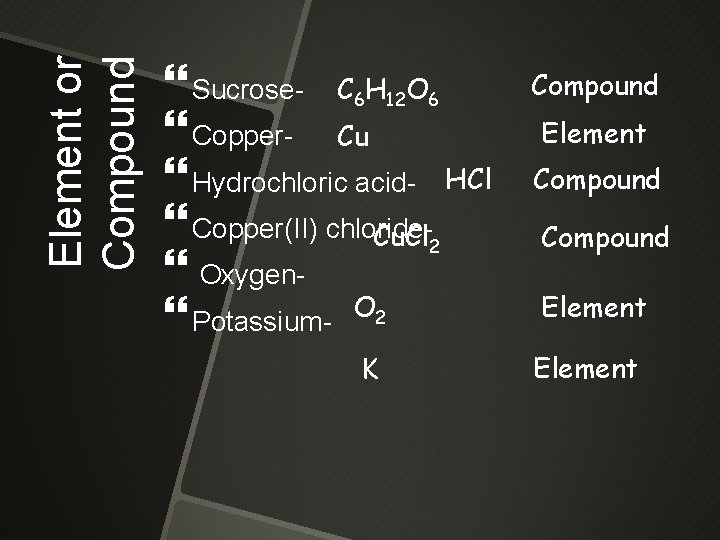
Element or Compound Sucrose- C 6 H 12 O 6 Copper- Cu Hydrochloric acid- HCl Copper(II) chloride. Cu. Cl 2 Oxygen Potassium- O 2 K Compound Element

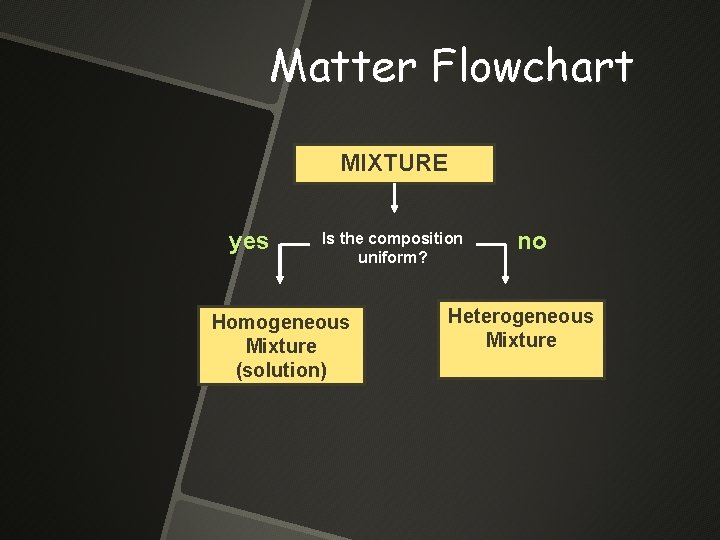
Matter Flowchart MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) no Heterogeneous Mixture

Mixtures Made up of two or more substances. Heterogeneous- mixture is not uniform in composition Hetero means different

Mi xt ure s Homogeneous- same composition throughout. Solution – a homogenous mixture with particles so small they cannot be seen with a microscope and will never settle to the bottom of their container Example: Vinegar, cola, apple juice

Alloys Alloy- homogeneous mixture composed of 2 or more metal elements Examples Steel – Fe , C, Cr, Ni Brass – Cu & Zn 14 K, 10 K, 18 K Gold

Homogeneous or Heterogeneous Homogeneous Milk. Heterogeneous Sugar water. Heterogeneous Trail mix Salt water solution- Homogeneous Steel. Homogeneous

Element, Compound, Heterogeneous, or Homogeneous Titanium Copper(II) sulfate Cheeseburger Calcium chloride Lithium nitrate solution- Calcium Acetone Brass- Element Compound Hetero Mixture Compound Homo Mixture Element Homo mixture-alloy

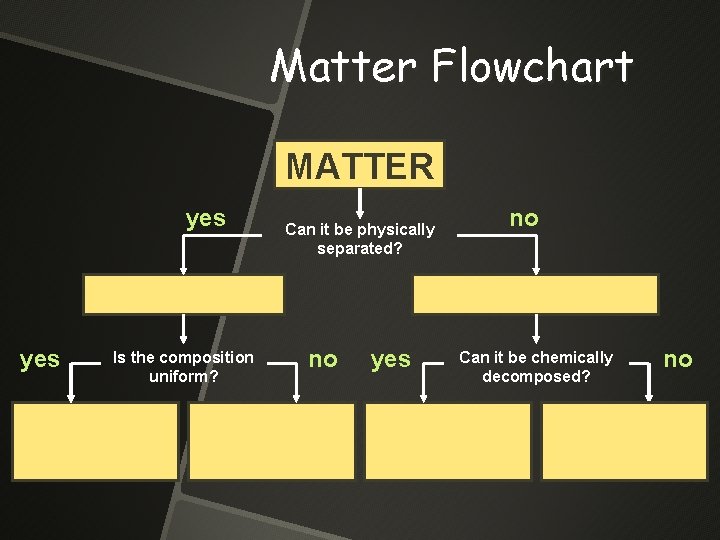
Matter Flowchart MATTER yes Is the composition uniform? Can it be physically separated? no yes no Can it be chemically decomposed? no

Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) no Can it be physically separated? PURE SUBSTANCE no Heterogeneous Mixture yes Can it be chemically decomposed? Compound no Element

Classwork Element, Compound, Mixture Worksheet Complete back of worksheet in class.

Se pa r a ti on Tec h n iqu e s Filtration-physical operation which is used for the separation of solids from fluids

Distillation

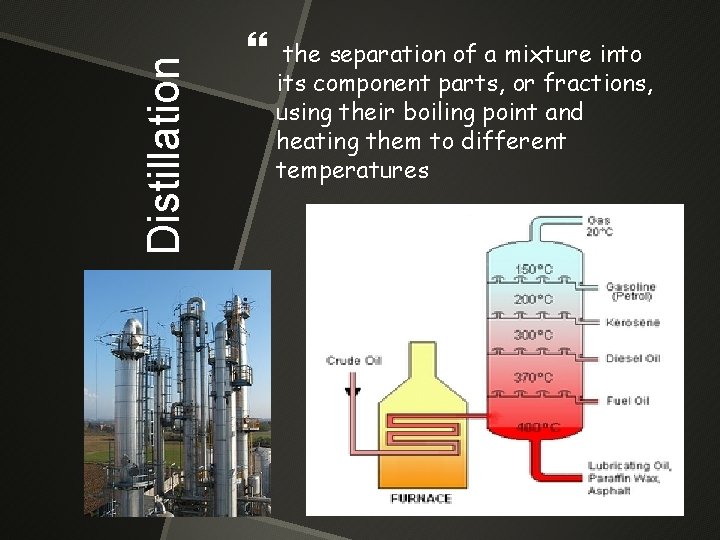
Fractional Distillation the separation of a mixture into its component parts, or fractions, using their boiling point and heating them to different temperatures

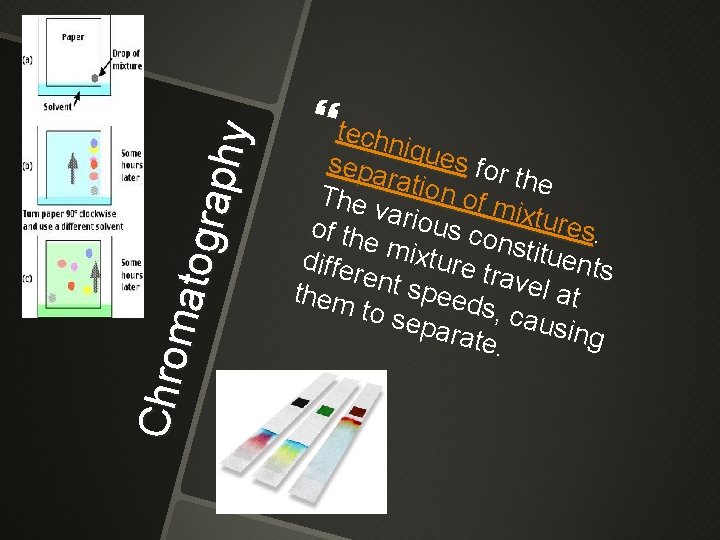
Chro mato grap hy techn sepa iques for the rat io n of m The vario ixture u of th e mi x s constit s. uent tur e differ s t rave ent s l at peed t hem s, c a to se usin para g t e.

Exit t i icket

Exit Ticket Sodium bicarbonate- Compound Kool-Aid- Homogeneous mixture 10 K gold- Homo mixture- alloy Helium gas- Element Sand, salt, and iron- Hetero mixture Sand- Compound Four corners- Move to a different corner of the classroom and determine each type of matter.
 What are the chemical change
What are the chemical change Absolute change and relative change formula
Absolute change and relative change formula Difference in physical and chemical changes
Difference in physical and chemical changes Supply and demand curve shifts
Supply and demand curve shifts Examples of physical vs chemical changes
Examples of physical vs chemical changes Rocks change due to temperature and pressure change
Rocks change due to temperature and pressure change Physical change and chemical change venn diagram
Physical change and chemical change venn diagram First order of change
First order of change Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 chemistry study guide
Chapter 10 chemistry study guide Chapter 1 review matter and change
Chapter 1 review matter and change Properties of and changes in matter grade 5
Properties of and changes in matter grade 5 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Chemistry matter and change chapter 2 answer key
Chemistry matter and change chapter 2 answer key Unit 2 matter and change
Unit 2 matter and change Circle the solute and underline the solvent
Circle the solute and underline the solvent Gray matter
Gray matter Label the cranial dura septa and associated sinuses.
Label the cranial dura septa and associated sinuses. Gray matter and white matter
Gray matter and white matter Pallium telencephalon
Pallium telencephalon Is cutting paper a physical change
Is cutting paper a physical change Integer number
Integer number Difference between supply and quantity supplied
Difference between supply and quantity supplied Change your water change your life
Change your water change your life Proactive vs reactive change
Proactive vs reactive change Spare change physical versus chemical change
Spare change physical versus chemical change When does a physical change occur study jams
When does a physical change occur study jams An example of a physical change
An example of a physical change Why is chopping wood a physical change
Why is chopping wood a physical change Climate change 2014 mitigation of climate change
Climate change 2014 mitigation of climate change Change in state of matter
Change in state of matter Two types of changes
Two types of changes Section 1 composition of matter
Section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Flow of energy vs flow of matter
Flow of energy vs flow of matter