Matter and Energy What is matter Matter and













- Slides: 33

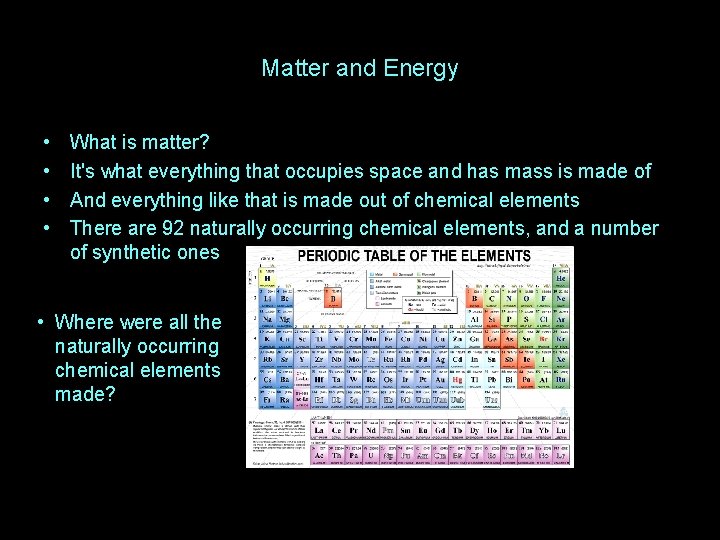
Matter and Energy • What is matter?

Matter and Energy • • What is matter? It's what everything that occupies space and has mass is made of And everything like that is made out of chemical elements There are 92 naturally occurring chemical elements, and a number of synthetic ones • Where were all the naturally occurring chemical elements made?

Matter and Energy • • What is matter? It's what everything that occupies space and has mass is made of And everything like that is made out of chemical elements There are 92 naturally occurring chemical elements, and a number of synthetic ones: • Where were all the naturally occurring chemical elements made? INSIDE STARS

Matter and Energy • • What is energy? It’s what makes things happen It’s what makes matter move We buy energy every day • What are some forms of energy you’ve bought this week? • So you can see that energy comes in a variety of forms

Matter and Energy • Forms of Energy – Kinetic energy -- energy of motion – Potential energy -- energy stored for later release as kinetic or radiative…there are several types: • gravitational • chemical • electrical • nuclear – Radiative energy -- energy carried by electromagnetic waves

Matter and Energy • The different forms of energy can be converted into one another • Understanding the conversions is essential to understanding astronomy • And this is tied to another fundamental conservation law: Conservation of Energy

Matter and Energy Conservation of Energy • In an isolated system (that can be as big as the universe) energy can change form, but the total amount never changes • Anything that happens involves an exchange of energy between material objects and/or the conversion of energy from one form to another. • Here's an example… • In science we like to quantify energy

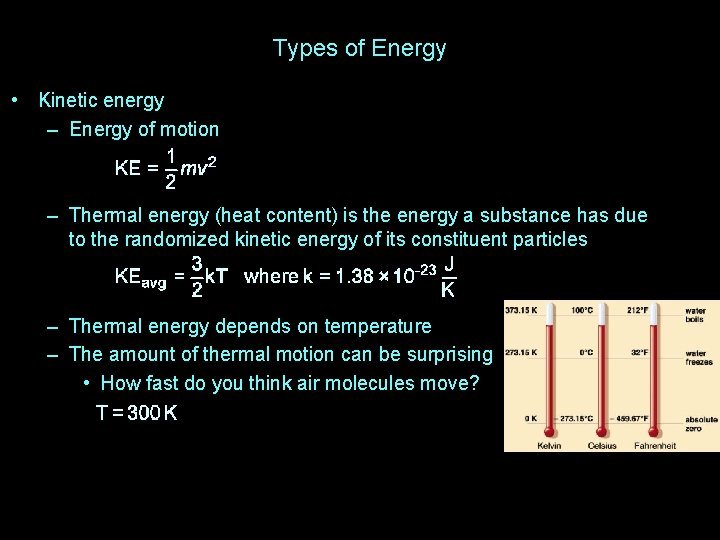
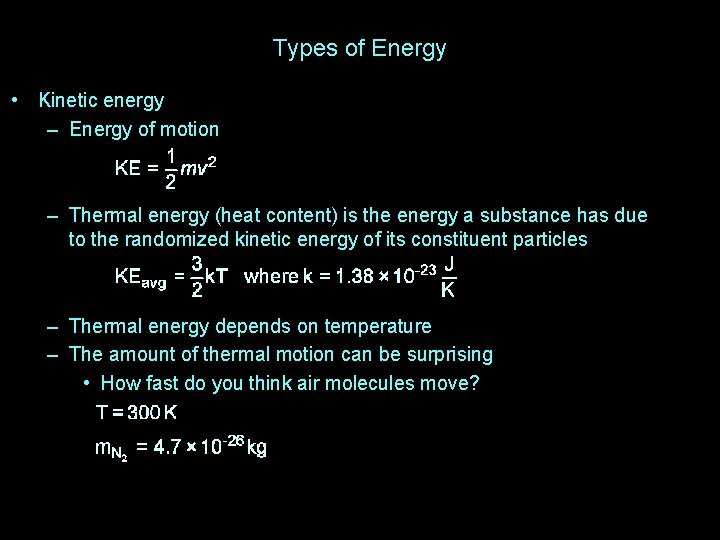
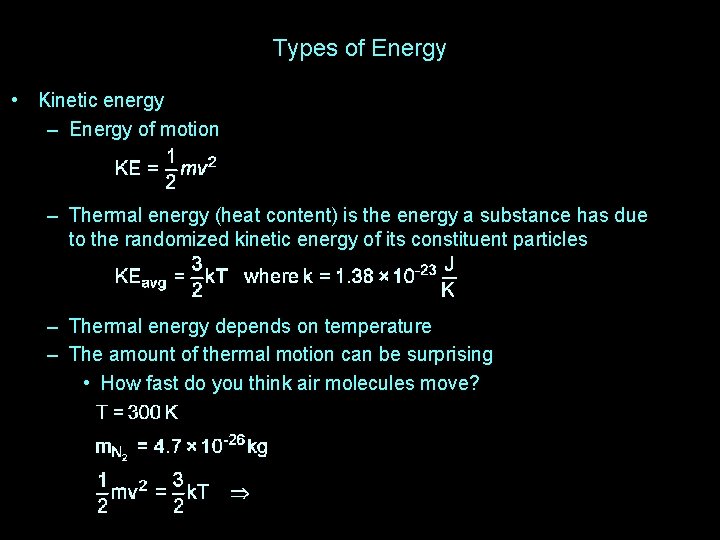
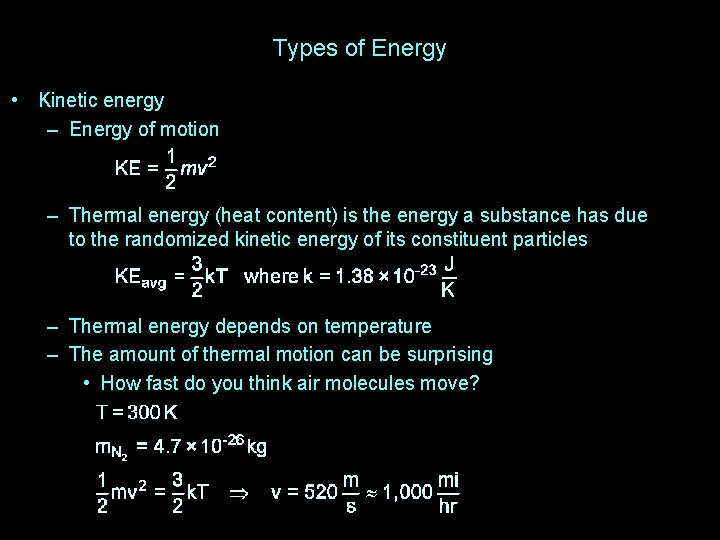
Types of Energy • Kinetic energy – Energy of motion – Thermal energy (heat content) is the energy a substance has due to the randomized kinetic energy of its constituent particles – Thermal energy depends on temperature – The amount of thermal motion can be surprising • How fast do you think air molecules move?

Types of Energy • Kinetic energy – Energy of motion – Thermal energy (heat content) is the energy a substance has due to the randomized kinetic energy of its constituent particles – Thermal energy depends on temperature – The amount of thermal motion can be surprising • How fast do you think air molecules move?

Types of Energy • Kinetic energy – Energy of motion – Thermal energy (heat content) is the energy a substance has due to the randomized kinetic energy of its constituent particles – Thermal energy depends on temperature – The amount of thermal motion can be surprising • How fast do you think air molecules move?

Types of Energy • Kinetic energy – Energy of motion – Thermal energy (heat content) is the energy a substance has due to the randomized kinetic energy of its constituent particles – Thermal energy depends on temperature – The amount of thermal motion can be surprising • How fast do you think air molecules move?

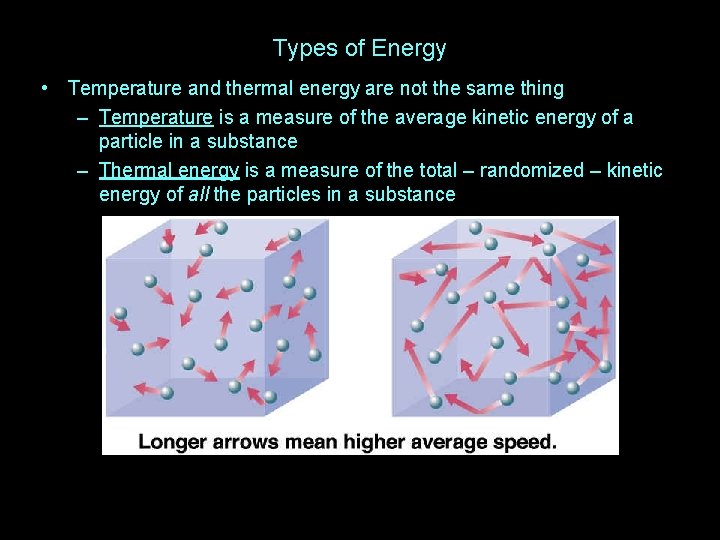
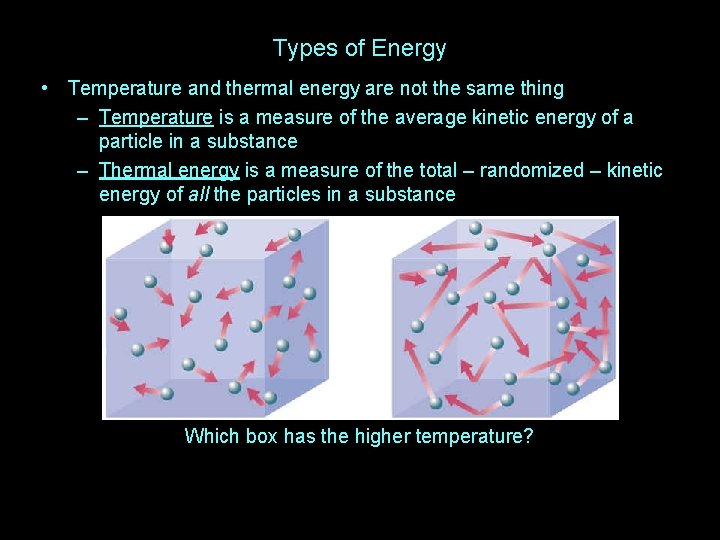
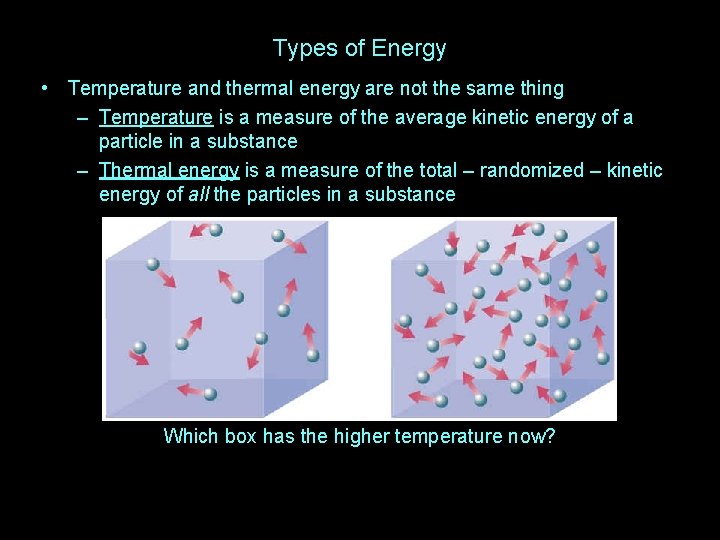
Types of Energy • Temperature and thermal energy are not the same thing

Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of a particle in a substance

Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of a particle in a substance – Thermal energy is a measure of the total – randomized – kinetic energy of all the particles in a substance

Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of a particle in a substance – Thermal energy is a measure of the total – randomized – kinetic energy of all the particles in a substance

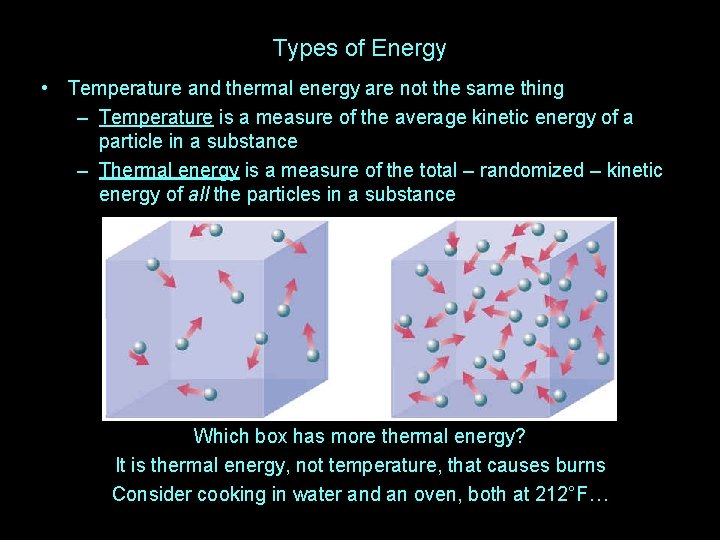
Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of a particle in a substance – Thermal energy is a measure of the total – randomized – kinetic energy of all the particles in a substance Which box has the higher temperature?

Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of a particle in a substance – Thermal energy is a measure of the total – randomized – kinetic energy of all the particles in a substance Which box has the higher temperature now?

Types of Energy • Temperature and thermal energy are not the same thing – Temperature is a measure of the average kinetic energy of a particle in a substance – Thermal energy is a measure of the total – randomized – kinetic energy of all the particles in a substance Which box has more thermal energy? It is thermal energy, not temperature, that causes burns Consider cooking in water and an oven, both at 212°F…

End lecture 100215

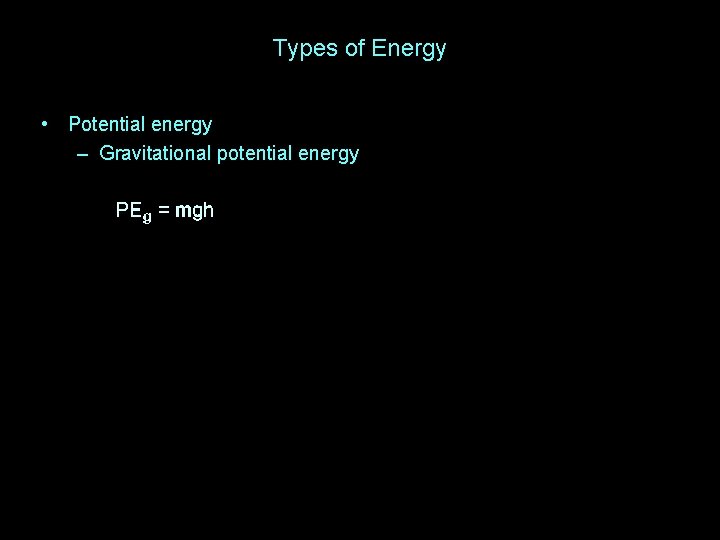
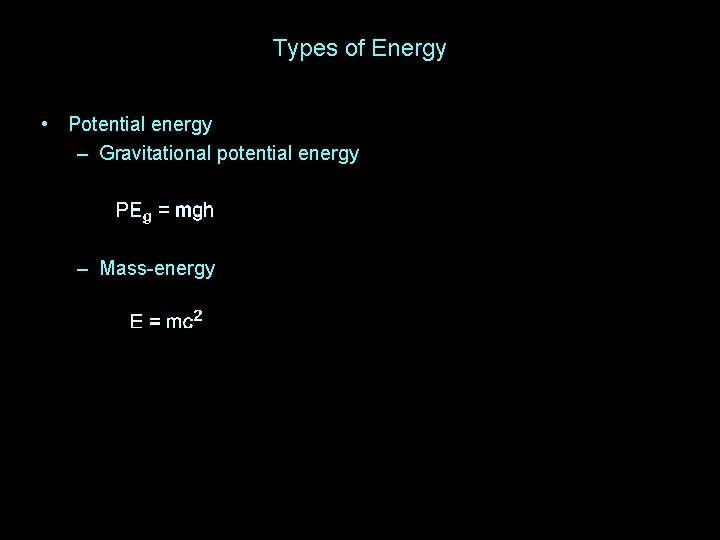
Types of Energy • Potential energy – Gravitational potential energy

Types of Energy • Potential energy – Gravitational potential energy – Mass-energy

Some Types of Energy Important in Astronomy • Kinetic energy – energy of motion • Thermal energy – randomized kinetic energy of collection of particles This is the average KE of a single particle • Gravitational potential energy – energy due to position in a gravity field • Mass-energy – energy equivalent of mass

• How much mass do you think is equivalent to the amount of energy released by metabolizing a candy bar – 1 x 106 J? – merely half a billionth of an ounce • How much is contained in 1 kg of mass? – 9 x 1016 J, the equivalent of an 18 megaton nuclear bomb, 1200 times the bomb that wiped out Hiroshima in 1945 – 1600 X more than is released by fissioning 1 kg of uranium!

The Material World • • Energy moves matter What is matter made of? It is composed of the chemical elements 92 naturally occurring chemical elements But there are many more substances than that. . . Why? Because atoms combine to form molecules and compounds These have very different properties than the elements they are made of: H 2 gas O 2 gas S solid H 2 O liquid H 2 SO 4 liquid

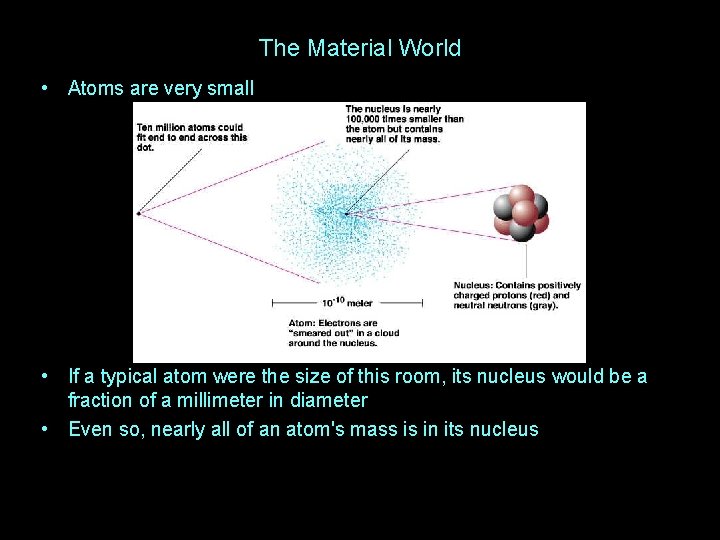
The Material World • Atoms are very small • If a typical atom were the size of this room, its nucleus would be a fraction of a millimeter in diameter • Even so, nearly all of an atom's mass is in its nucleus

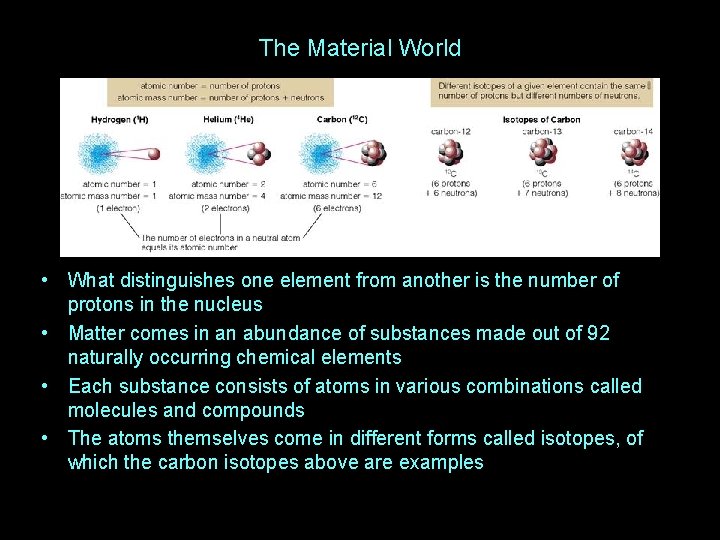
The Material World • What distinguishes one element from another is the number of protons in the nucleus • Matter comes in an abundance of substances made out of 92 naturally occurring chemical elements • Each substance consists of atoms in various combinations called molecules and compounds • The atoms themselves come in different forms called isotopes, of which the carbon isotopes above are examples

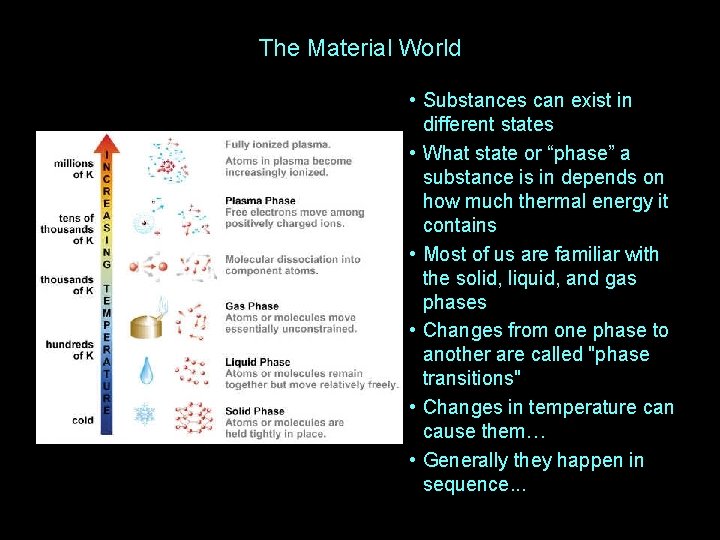
The Material World • Substances can exist in different states • What state or “phase” a substance is in depends on how much thermal energy it contains • Most of us are familiar with the solid, liquid, and gas phases • Changes from one phase to another are called "phase transitions" • Changes in temperature can cause them… • Generally they happen in sequence. . .

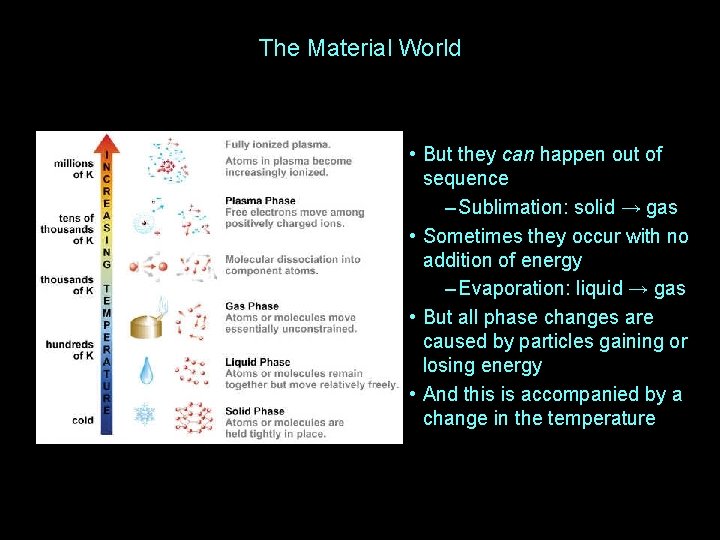
The Material World • But they can happen out of sequence – Sublimation: solid → gas • Sometimes they occur with no addition of energy – Evaporation: liquid → gas • But all phase changes are caused by particles gaining or losing energy • And this is accompanied by a change in the temperature

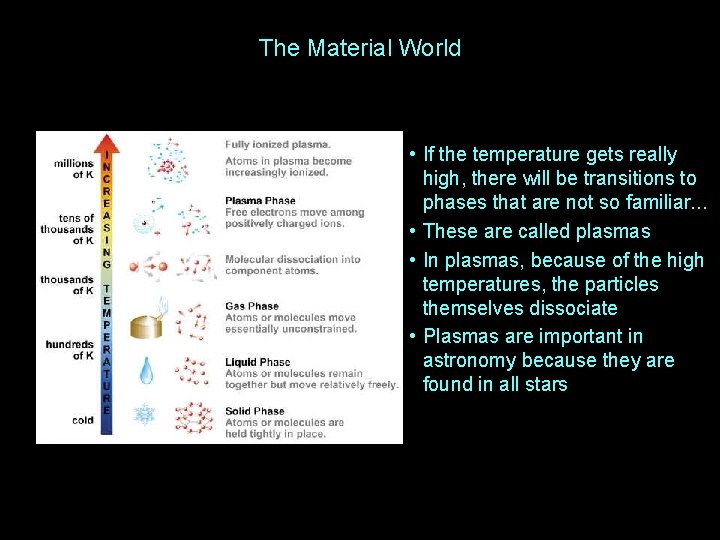
The Material World • If the temperature gets really high, there will be transitions to phases that are not so familiar. . . • These are called plasmas • In plasmas, because of the high temperatures, the particles themselves dissociate • Plasmas are important in astronomy because they are found in all stars

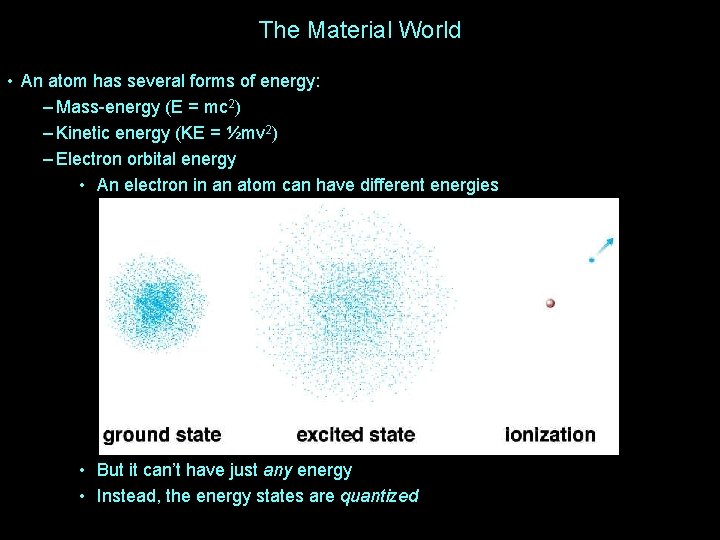
The Material World • An atom has several forms of energy: – Mass-energy (E = mc 2) – Kinetic energy (KE = ½mv 2) – Electron orbital energy • An electron in an atom can have different energies • But it can’t have just any energy • Instead, the energy states are quantized

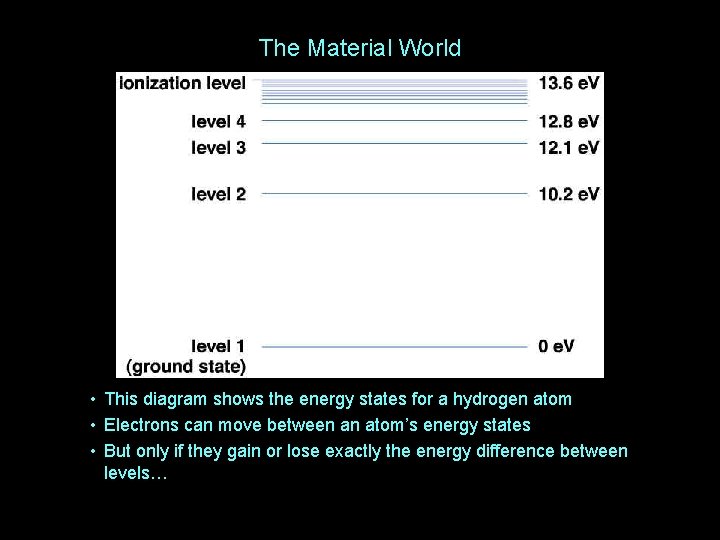
The Material World • This diagram shows the energy states for a hydrogen atom • Electrons can move between an atom’s energy states • But only if they gain or lose exactly the energy difference between levels…

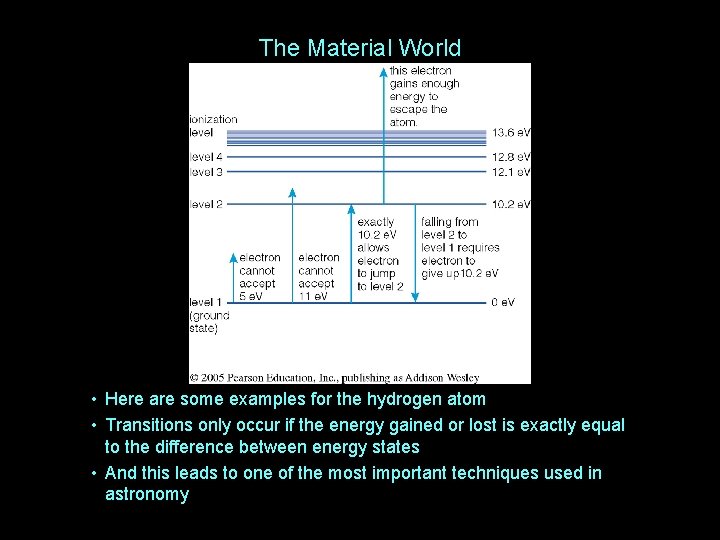
The Material World • Here are some examples for the hydrogen atom • Transitions only occur if the energy gained or lost is exactly equal to the difference between energy states • And this leads to one of the most important techniques used in astronomy

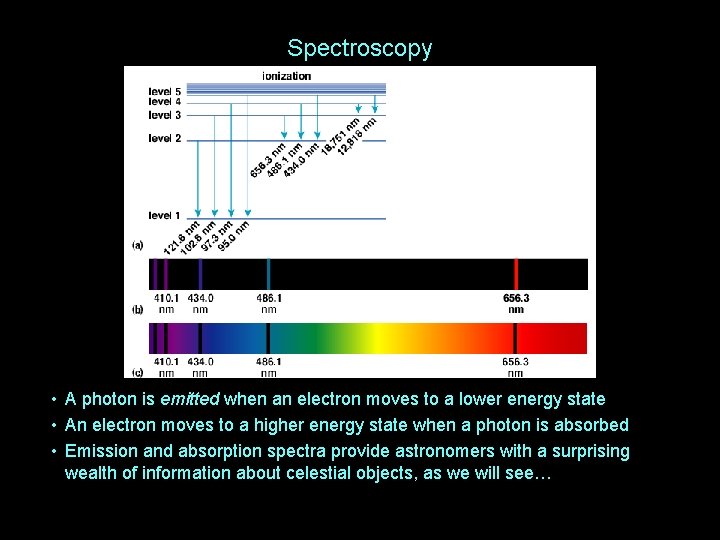
Spectroscopy • A photon is emitted when an electron moves to a lower energy state • An electron moves to a higher energy state when a photon is absorbed • Emission and absorption spectra provide astronomers with a surprising wealth of information about celestial objects, as we will see…
 Energy naturally flows from warmer matter to cooler matter.
Energy naturally flows from warmer matter to cooler matter. Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Grey vs white matter
Grey vs white matter Median and lateral apertures
Median and lateral apertures Gray matter and white matter
Gray matter and white matter Telencephalon
Telencephalon Lesson 3 energy and matter in ecosystems answer key
Lesson 3 energy and matter in ecosystems answer key Which reverses the normal flow of thermal energy
Which reverses the normal flow of thermal energy Section 1 matter and thermal energy
Section 1 matter and thermal energy Science matter and energy
Science matter and energy Matter energy and measurement
Matter energy and measurement Dark matter and dark energy ppt
Dark matter and dark energy ppt Unit 2 matter and energy
Unit 2 matter and energy List the secondary consumers
List the secondary consumers Lesson 1 thermal energy and the behavior of matter
Lesson 1 thermal energy and the behavior of matter Phase change concept map solid liquid gas
Phase change concept map solid liquid gas Whats the study of matter and energy
Whats the study of matter and energy Examples of mechanical waves
Examples of mechanical waves Kesler science properties of waves answer key
Kesler science properties of waves answer key Energy pyramid labeled
Energy pyramid labeled Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter What is the definition of chemical potential energy
What is the definition of chemical potential energy Primary energy and secondary energy
Primary energy and secondary energy Primary energy and secondary energy
Primary energy and secondary energy Helmholtz free energy and gibbs free energy
Helmholtz free energy and gibbs free energy Renewable energy and energy efficiency partnership
Renewable energy and energy efficiency partnership Potential energy summary
Potential energy summary Spring potential and kinetic energy
Spring potential and kinetic energy Gravitational potential energy
Gravitational potential energy Potential energy units
Potential energy units