Unit 2 Matter and Energy Chemistry is the

























- Slides: 48

Unit 2 Matter and Energy

Chemistry is… …the study of the composition, structure, and properties of matter and the changes it undergoes Matter Anything that has mass and occupies space (volume)

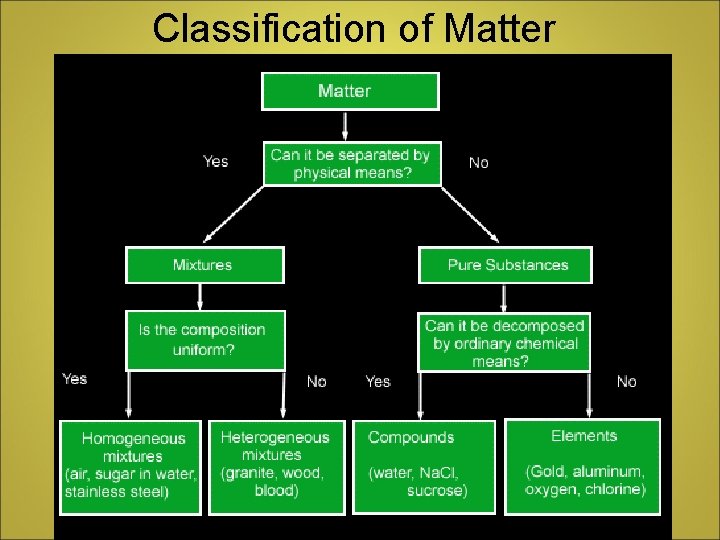
Classification of Matter

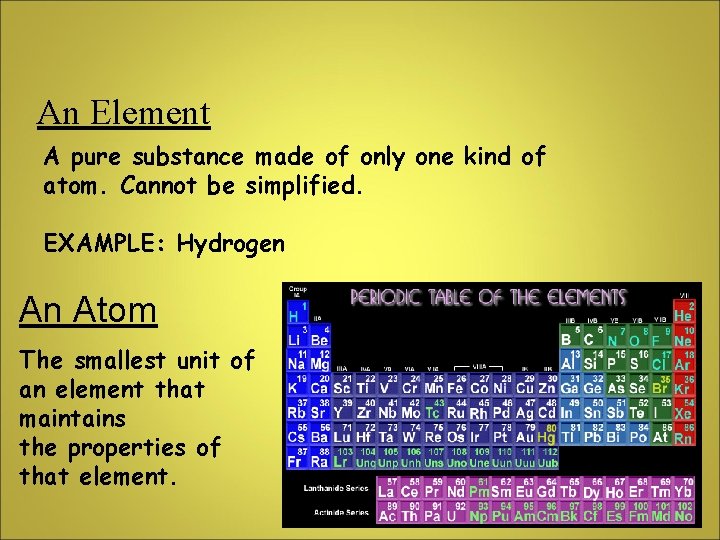
An Element A pure substance made of only one kind of atom. Cannot be simplified. EXAMPLE: Hydrogen An Atom The smallest unit of an element that maintains the properties of that element.

Element Song! Tom Lehrer: http: //youtu. be/DYW 50 F 42 ss 8 Harry Potter: http: //youtu. be/v 1 Tf. PDl. A 1 x. E

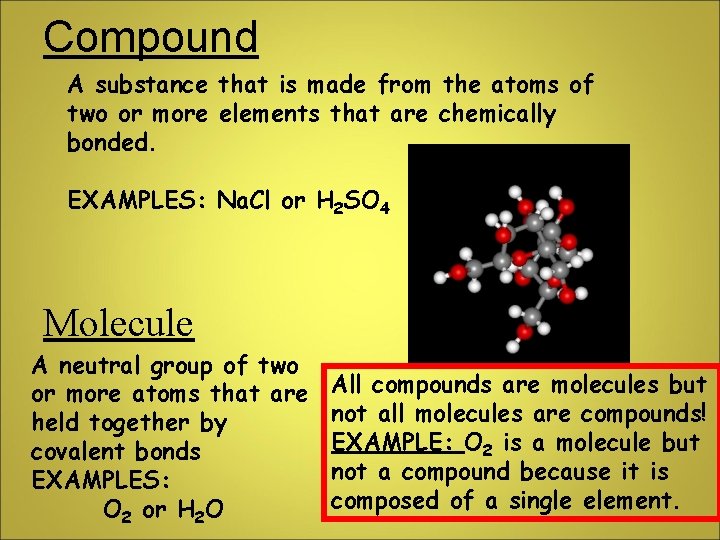
Compound A substance that is made from the atoms of two or more elements that are chemically bonded. EXAMPLES: Na. Cl or H 2 SO 4 Molecule A neutral group of two or more atoms that are held together by covalent bonds EXAMPLES: O 2 or H 2 O All compounds are molecules but not all molecules are compounds! EXAMPLE: O 2 is a molecule but not a compound because it is composed of a single element.

Are you ready for this? An imperfect analogy… Imagine going to an ice cream store. Let's say that they have 30 different flavors of ice cream. Those are elements, the things that I have available to build my dessert from. The smallest amount of ice cream that the store will sell to me is a scoop. This is an atom. If I want, I can put two or more scoops of ice cream together. This is a molecule. If my molecule has more than one flavor of ice cream, I can call it a compound.

Reading Compounds • Subscripts: – The little number to the right of the element in the compound – Tell you the number of atoms in each compound • Coefficients: – The large number before the compound – Tells you how many molecules of the compounds you have

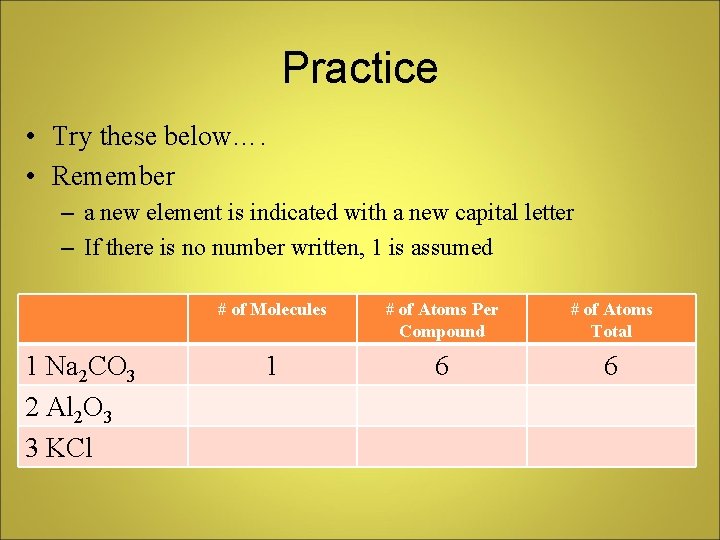
Practice • Try these below…. • Remember – a new element is indicated with a new capital letter – If there is no number written, 1 is assumed 1 Na 2 CO 3 2 Al 2 O 3 3 KCl # of Molecules # of Atoms Per Compound # of Atoms Total 1 6 6

Laws About Compounds • Law of Definite Composition – A pure compound has the same ratio of atoms always • Ex. Water is always H 2 O • Law of Multiple Proportions – Elements can combine in different ratios to give different compounds • Ex. Hydrogen Peroxide H 2 O 2

Mixtures Two or more substances/matter which are mixed but are not combined chemically. Each retains its own properties. -Despite that there are no chemical changes to its components, the physical properties of a mixture may differ from those of the components. Such as melting point. EXAMPLE: adding salt to water changes the boiling point and melting point Therefore, why do we add salt to roads in the winter?

EXAMPLES OF MIXTURES HETEROGENOUS -not uniform in composition EXAMPLE: Salad dressing HOMOGENOUS -uniform in composition EXAMPLE: Water

Heterogeneous vs. Homogeneous Milk HOMOGENEOUS

Heterogeneous vs. Homogeneous Blueberry Muffin HETEROGENEOUS

Heterogeneous vs. Homogeneous Water HOMOGENEOUS

Heterogeneous vs. Homogeneous Ice Water HETEROGENEOUS

Heterogeneous vs. Homogeneous Orange Juice (with pulp) HE TE RO GE NE OU S

Heterogeneous vs. Homogeneous Orange Juice (without pulp) U N E H O G O M O E S

Heterogeneous vs. Homogeneous Air HOMOGENEOUS

Heterogeneous vs. Homogeneous Water and Oil HETEROGENEOUS

Heterogeneous vs. Homogeneous Trail Mix HETEROGENEOUS

Heterogeneous vs. Homogeneous Soda (aka Coke) HETEROGENEOUS

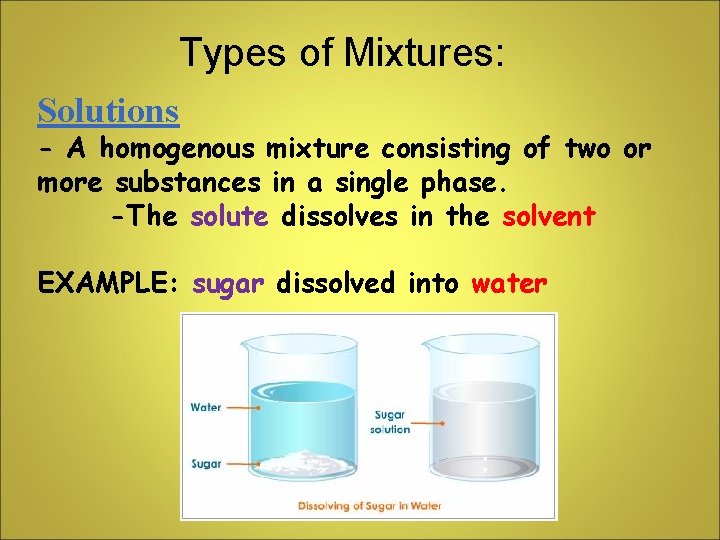
Types of Mixtures: Solutions - A homogenous mixture consisting of two or more substances in a single phase. -The solute dissolves in the solvent EXAMPLE: sugar dissolved into water

Types of Mixtures: Suspension -mixture of two or more ingredients, where the particles are typically large enough to be seen Solid-Solid-Liquid-Liquid

Types of Mixtures: Colloid -A substance microscopically dispersed throughout another substance. Can be a solid, liquid, or gas. Example: milk jell-o

Separation of a Mixture The constituents of the mixture retain their identity and may be separated by physical means (distillation, chromatography, filtration, vaporization).

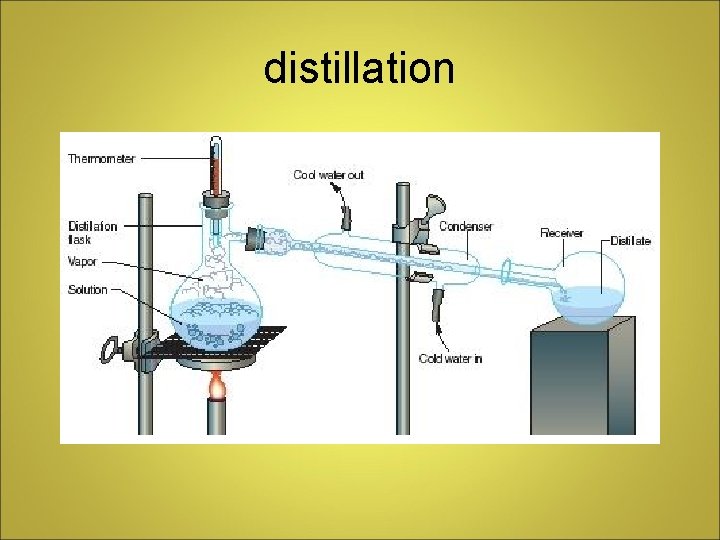
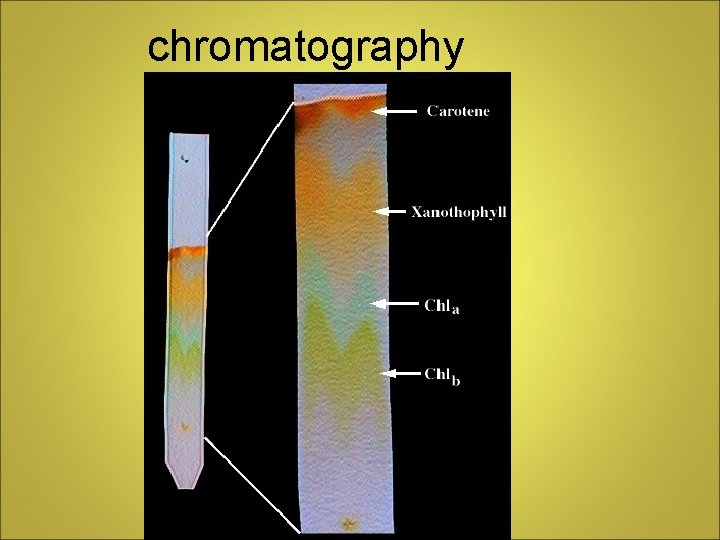
Separating Mixtures • Filtration - porous barrier separates solid from liquid • Distillation - liquids separated by differences in boiling point • Crystallization - forms pure solids from dissolved substances • Chromatography - separation based on ability to travel or be drawn across a material

filtration

distillation

crystallization

chromatography

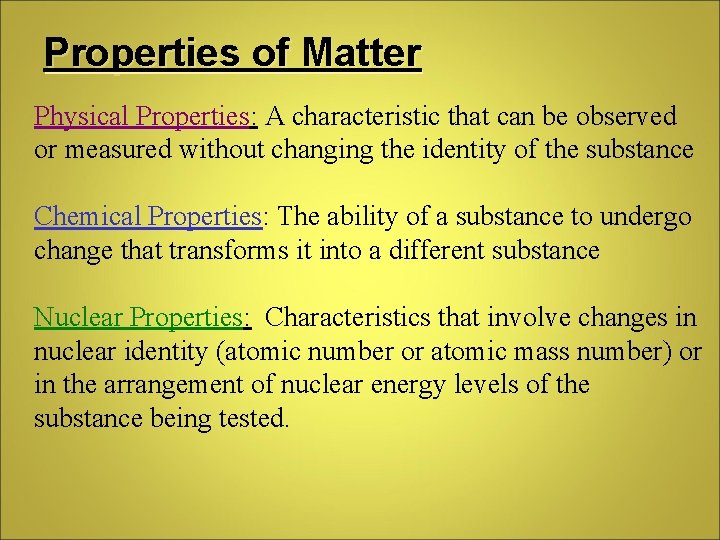
Properties of Matter Physical Properties: A characteristic that can be observed or measured without changing the identity of the substance Chemical Properties: The ability of a substance to undergo change that transforms it into a different substance Nuclear Properties: Characteristics that involve changes in nuclear identity (atomic number or atomic mass number) or in the arrangement of nuclear energy levels of the substance being tested.

Which is which? Physical or Chemical property? 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. melting point solubility boiling point reactivity freezing point density ability to oxidize color odor flammability combustibility malleability toxicity 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Physical Physical Chemical Physical Chemical

Physical Properties of Matter Can be Extensive or Intensive Extensive properties depend on the amount of matter that is present. Intensive properties does not depend on the amount of matter present.

Which is which? Extensive or Intensive property? 1. 2. 3. 4. 5. 6. 7. 8. 9. Volume Boiling point Energy content Density Melting point Mass Ductility Length Elasticity 1. 2. 3. 4. 5. 6. 7. 8. 9. Extensive Intensive Extensive Intensive

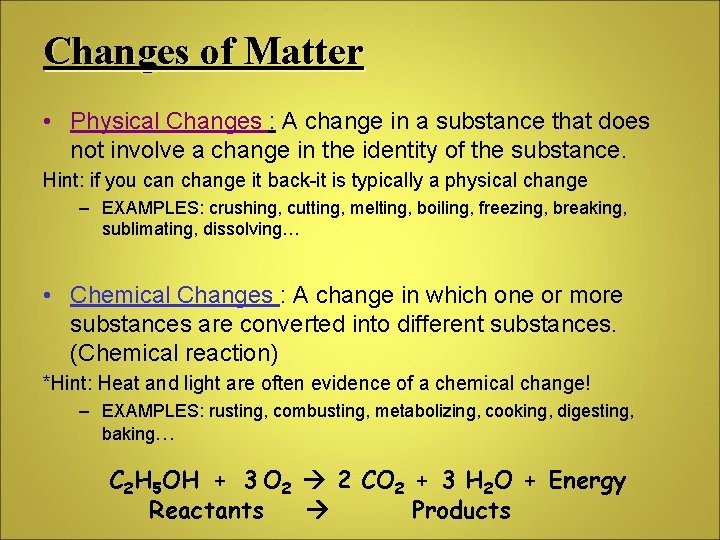
Changes of Matter • Physical Changes : A change in a substance that does not involve a change in the identity of the substance. Hint: if you can change it back-it is typically a physical change – EXAMPLES: crushing, cutting, melting, boiling, freezing, breaking, sublimating, dissolving… • Chemical Changes : A change in which one or more substances are converted into different substances. (Chemical reaction) *Hint: Heat and light are often evidence of a chemical change! – EXAMPLES: rusting, combusting, metabolizing, cooking, digesting, baking… C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O + Energy Reactants Products

Which is which? Physical or Chemical change? 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. Water boiling Ice melting Cutting your hair An acid neutralizing a base Breaking a piece of glass Cooking an egg Your bike rusting Sublimating dry ice Casting silver in a mold Wood burning Digesting food Bending melt to form a bridge Metabolizing sugar Mixing red and blue marbles Milk sours Water freezing Dissolving sugar into water 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. Physical Chemical Physical Chemical Physical

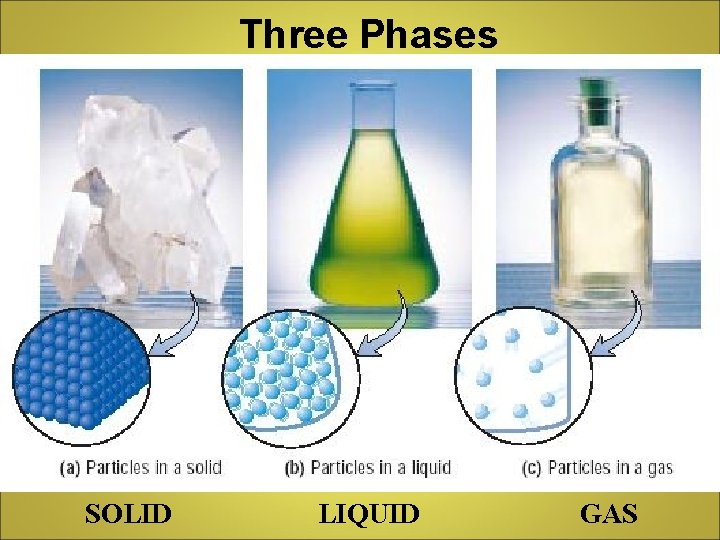
Three Phases SOLID LIQUID GAS

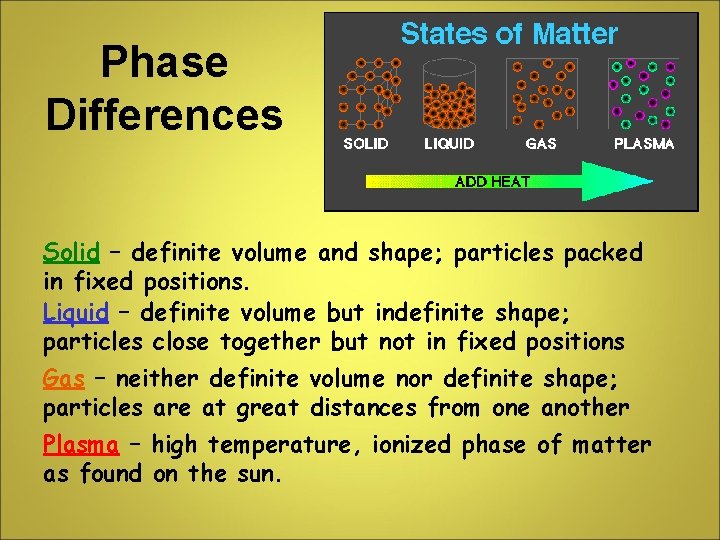
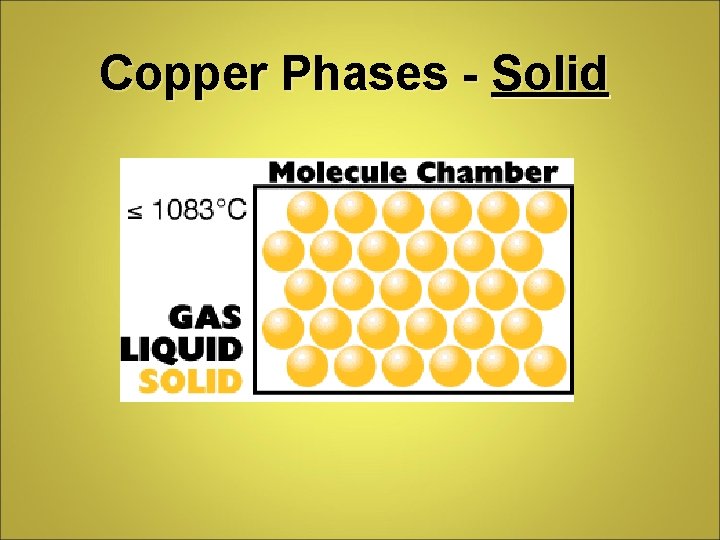
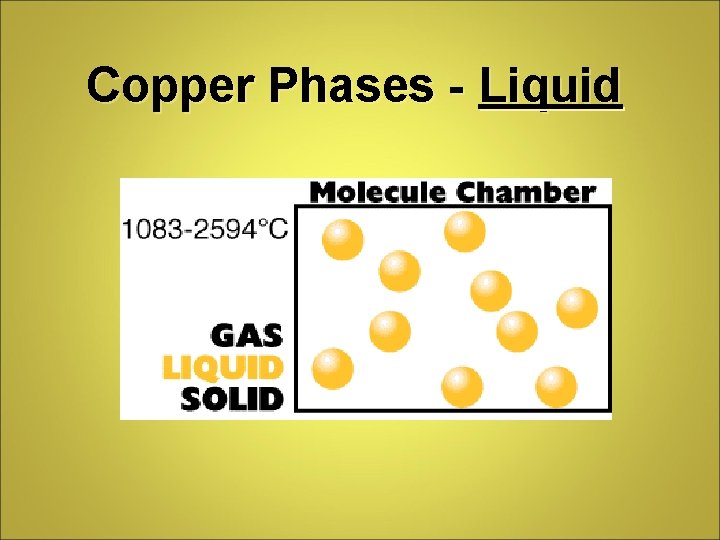
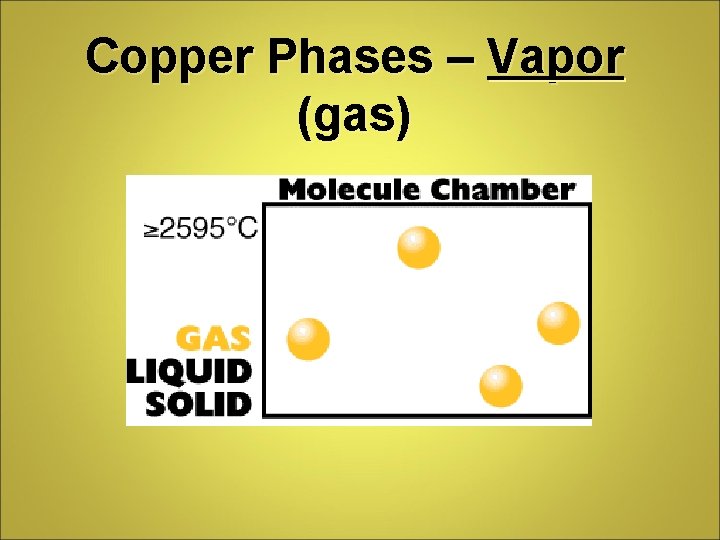
Phase Differences Solid – definite volume and shape; particles packed in fixed positions. Liquid – definite volume but indefinite shape; particles close together but not in fixed positions Gas – neither definite volume nor definite shape; particles are at great distances from one another Plasma – high temperature, ionized phase of matter as found on the sun.

Copper Phases - Solid

Copper Phases - Liquid

Copper Phases – Vapor (gas)

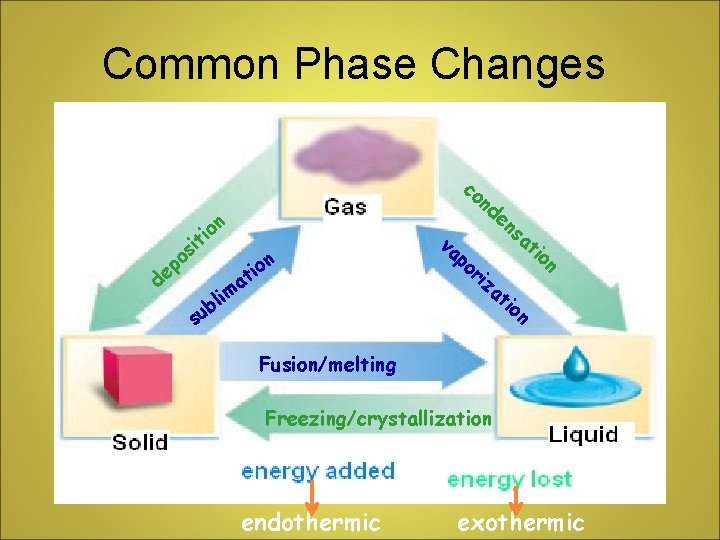
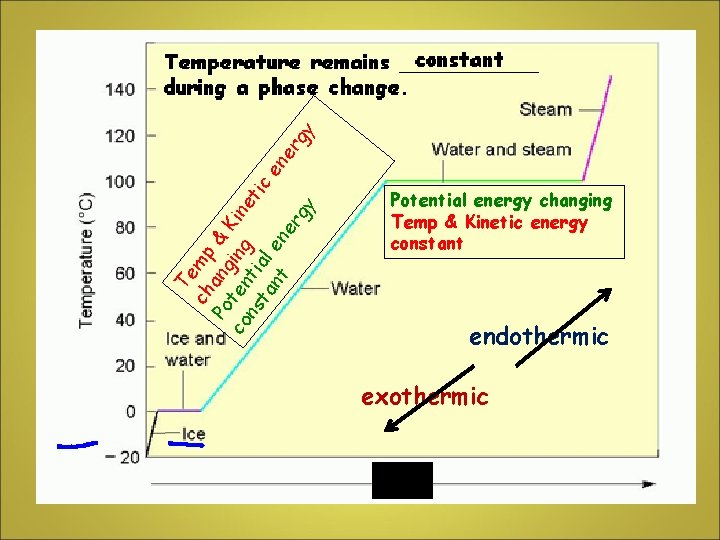
PHASE CHANGE • Involves PHYSICAL CHANGES from solid to liquid, liquid to solid, liquid to gas, etc • Particles absorb or release heat during phase changes NOTE: THE TEMPERATURE OF THE MATTER DOES NOT CHANGE DURING A PHASE CHANGE!

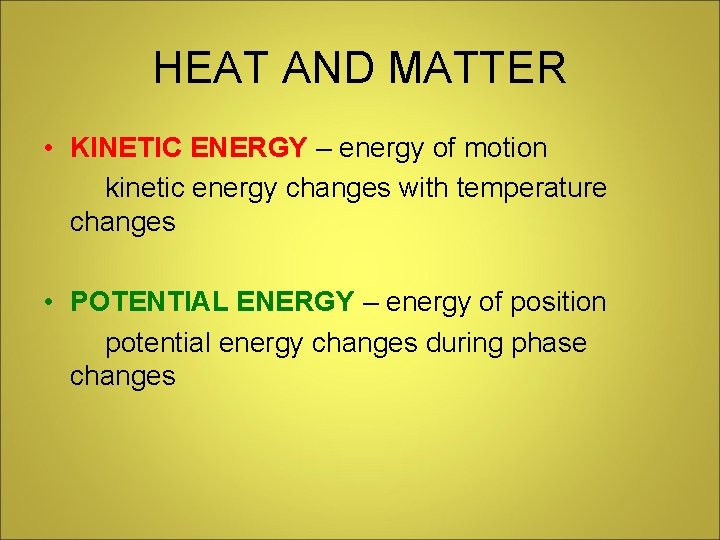
HEAT AND MATTER • KINETIC ENERGY – energy of motion kinetic energy changes with temperature changes • POTENTIAL ENERGY – energy of position potential energy changes during phase changes

Common Phase Changes co on i it s o ep nd d n o i t a il m ub va po ri en za sa ti on s ti on Fusion/melting Freezing/crystallization endothermic exothermic

Te ch mp Po ang & co ten ing Kine ns ti tic ta al en nt en er er gy gy Potential energy changing Temp & Kinetic energy constant endothermic exothermic h

HEAT ENERGY • Energy is absorbed or released as heat in chemical or physical changes – energy involved can be measured • DURING PHYSICAL/CHEMICAL CHANGES ENERGY AND MATTER ARE CONSERVED! • Law of Conservation of Energy- Energy is not created or destroyed-only changes forms • Heat energy impacts particle motion (atoms, ions, molecules) – more heat, more motion; less heat, less motion

Kinetic–Molecular Theory of Matter • Developed in late 19 th century to account for behavior of atoms and molecules • Based on idea that particles of matter are always in motion, and have more energy as the temperature increases: KINETIC ENERGY = ENERGY OF MOTION • Used to explain properties of solids, liquids & gases in terms of the energy of particles and forces that act between them
 Flow energy review
Flow energy review Unit 2 matter and energy
Unit 2 matter and energy Grey matter nervous system
Grey matter nervous system Gyrus and sulcus function
Gyrus and sulcus function Gray matter and white matter
Gray matter and white matter Rhinencephalon
Rhinencephalon Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Chemistry matter and its changes
Chemistry matter and its changes Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 chemistry study guide
Chapter 10 chemistry study guide Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chapter 10 study guide the mole
Chapter 10 study guide the mole Chemistry matter and change answer key chapter 2
Chemistry matter and change answer key chapter 2 Section 1 composition of matter
Section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Composition of matter section 1
Composition of matter section 1 Examples of matter in chemistry
Examples of matter in chemistry Flowchart of matter
Flowchart of matter Matter flowchart chemistry
Matter flowchart chemistry Graphic organizer classification of matter
Graphic organizer classification of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Basic food chemistry the nature of matter
Basic food chemistry the nature of matter Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Lesson 3 energy and matter in ecosystems answer key
Lesson 3 energy and matter in ecosystems answer key Section 16.1 thermal energy and matter
Section 16.1 thermal energy and matter Section 1 matter and thermal energy
Section 1 matter and thermal energy Science matter
Science matter Matter energy and measurement
Matter energy and measurement Dark matter and dark energy ppt
Dark matter and dark energy ppt Trophic levels.
Trophic levels. The science duo physical and chemical changes
The science duo physical and chemical changes Phase change concept map
Phase change concept map States of matter foldable
States of matter foldable Example of mechanical wave
Example of mechanical wave Kesler science properties of waves answer key
Kesler science properties of waves answer key Heterotroph on energy pyramid
Heterotroph on energy pyramid Unit 6 review questions
Unit 6 review questions Unit 2 matter and change
Unit 2 matter and change Ap chem spontaneity entropy and free energy
Ap chem spontaneity entropy and free energy Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào