Matter Energy and Measurement Chapter 1 The Scientific














































- Slides: 75

Matter, Energy, and Measurement Chapter 1

The Scientific Approach to Knowledge • philosophers try to understand the universe by reasoning and thinking about “ideal” behavior • scientists try to understand the universe through empirical knowledge gained through observation and experiment Tro, Chemistry: A Molecular Approach 2

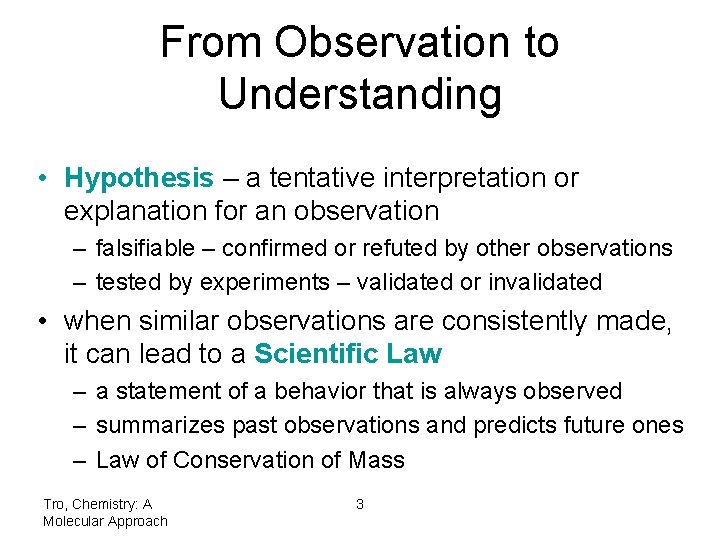
From Observation to Understanding • Hypothesis – a tentative interpretation or explanation for an observation – falsifiable – confirmed or refuted by other observations – tested by experiments – validated or invalidated • when similar observations are consistently made, it can lead to a Scientific Law – a statement of a behavior that is always observed – summarizes past observations and predicts future ones – Law of Conservation of Mass Tro, Chemistry: A Molecular Approach 3

From Specific to General Understanding • a hypothesis is a potential explanation for a single or small number of observations • a theory is a general explanation for the manifestation and behavior of all nature – models – pinnacle of scientific knowledge – validated or invalidated by experiment and observation Tro, Chemistry: A Molecular Approach 4

Classification of Matter States of Matter Physical and Chemical Properties Physical and Chemical Changes

Classification of Matter • matter is anything that has mass and occupies space • we can classify matter based on whether it’s solid, liquid, or gas Tro, Chemistry: A Molecular Approach 6

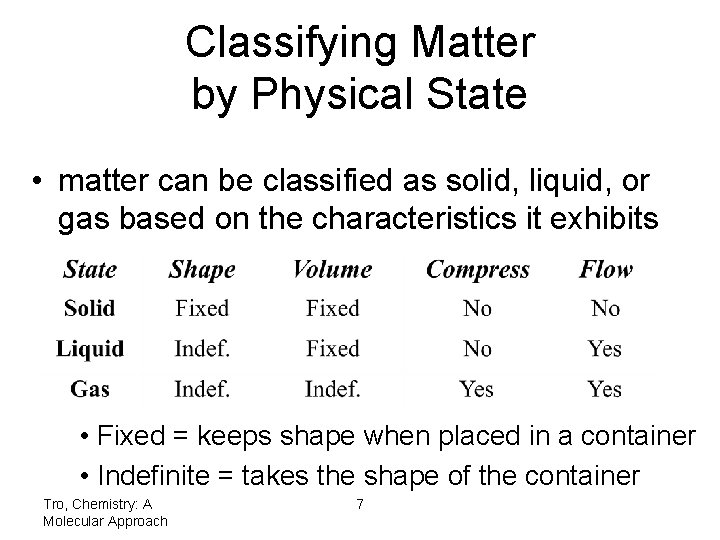
Classifying Matter by Physical State • matter can be classified as solid, liquid, or gas based on the characteristics it exhibits • Fixed = keeps shape when placed in a container • Indefinite = takes the shape of the container Tro, Chemistry: A Molecular Approach 7

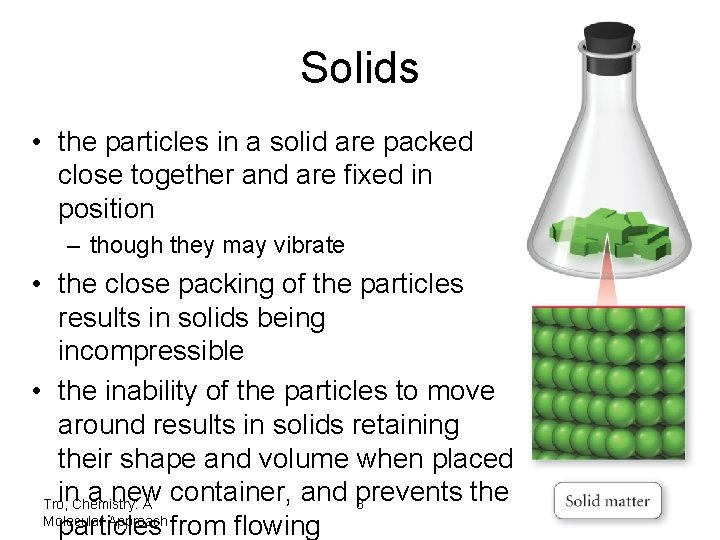
Solids • the particles in a solid are packed close together and are fixed in position – though they may vibrate • the close packing of the particles results in solids being incompressible • the inability of the particles to move around results in solids retaining their shape and volume when placed in a new container, and prevents the Tro, Chemistry: A 8 Molecular Approach particles from flowing

Liquids • the particles in a liquid are closely packed, but they have some ability to move around • the close packing results in liquids being incompressible • but the ability of the particles to move allows liquids to take the shape of their container and to flow – however, they don’t have enough freedom to escape and expand to fill the container Tro, Chemistry: A Molecular Approach 9

Gases • in the gas state, the particles have complete freedom from each other • the particles are constantly flying around, bumping into each other and the container • in the gas state, there is a lot of empty space between the particles – on average Tro, Chemistry: A Molecular Approach 10

Changes of State • • • Solid to Liquid = Melting Liquid to Solid = Freezing Liquid to Gas = Evaporation Gas to Liquid = Condensation Solid to Gas = Sublimation (e. g. dry ice) Gas to Solid = Deposition

Changes in Matter • changes that alter the state or appearance of the matter without altering the composition are called physical changes • changes that alter the composition of the matter are called chemical changes – during the chemical change, the atoms that are present rearrange into new molecules, but all of the original atoms are still present Tro, Chemistry: A Molecular Approach 12

Physical Changes – changes in the physical appearance of a substance, still have the same substance after change e. g. • ripping up a piece of paper • Melting an ice cube • Freezing water • Boiling water

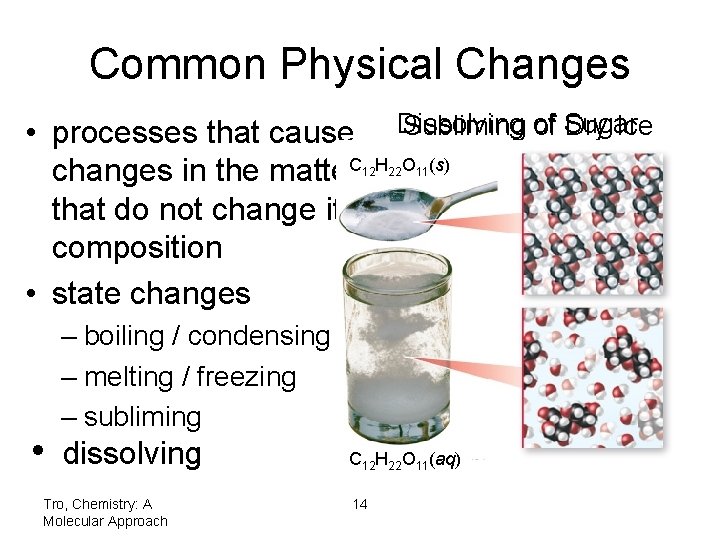
Common Physical Changes • processes that cause changes in the matter. C that do not change its composition • state changes Dissolving Subliming of Sugar Dry Ice 12 H 22 O 11(s) CO 2(g) Dry Ice – boiling / condensing – melting / freezing – subliming • dissolving Tro, Chemistry: A Molecular Approach CO 2(s) C 12 H 22 O 11(aq) 14

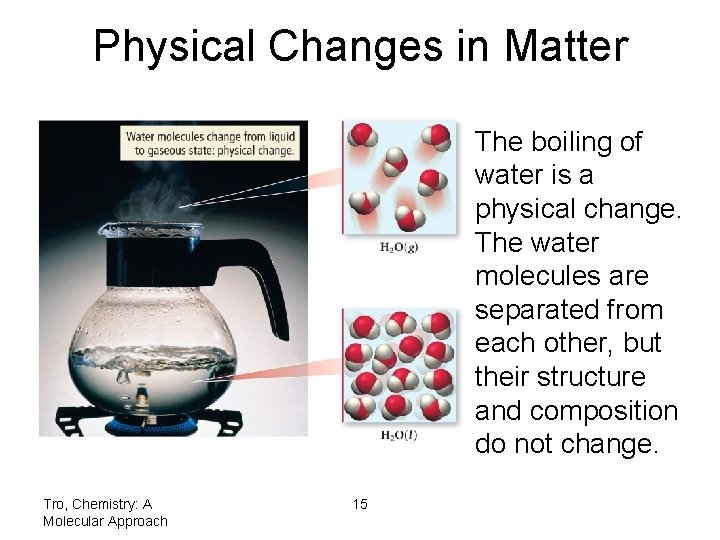
Physical Changes in Matter The boiling of water is a physical change. The water molecules are separated from each other, but their structure and composition do not change. Tro, Chemistry: A Molecular Approach 15

Changes in Matter Chemical Changes – involve a chemical reaction, have different substance after the change than had before. e. g. • water decomposing into oxygen and hydrogen • Gasoline burning in air to produce carbon dioxide and water

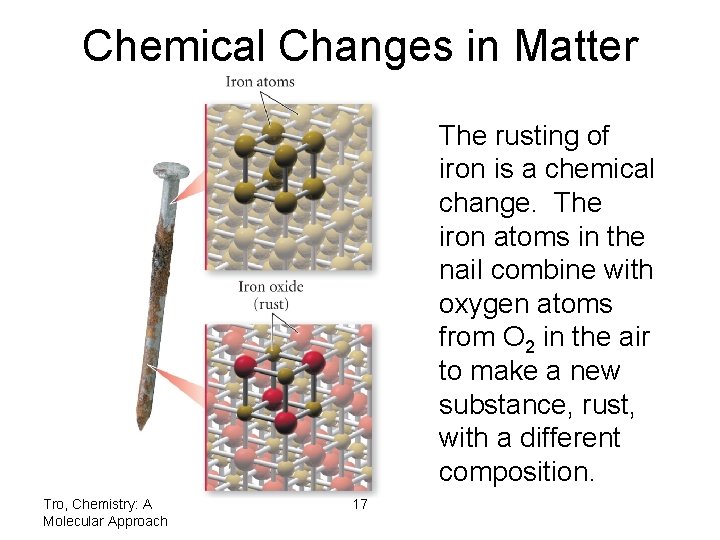
Chemical Changes in Matter The rusting of iron is a chemical change. The iron atoms in the nail combine with oxygen atoms from O 2 in the air to make a new substance, rust, with a different composition. Tro, Chemistry: A Molecular Approach 17

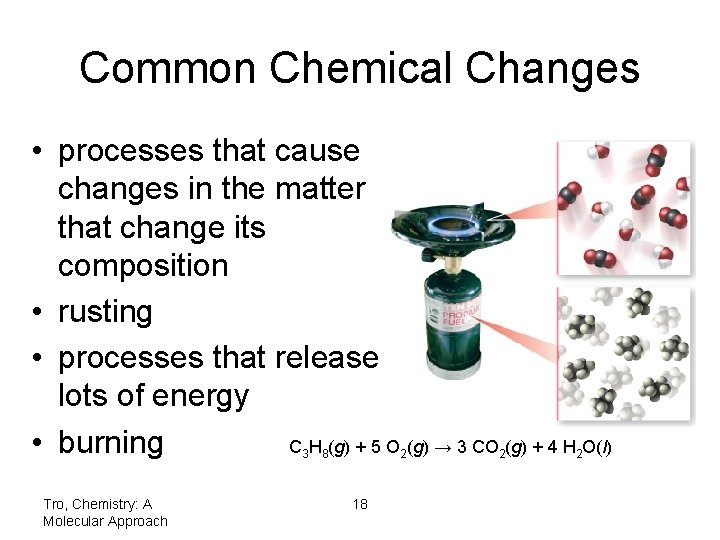
Common Chemical Changes • processes that cause changes in the matter that change its composition • rusting • processes that release lots of energy • burning C H (g) + 5 O (g) → 3 CO (g) + 4 H O(l) 3 Tro, Chemistry: A Molecular Approach 8 2 18 2 2

Properties of Matter • physical properties are the characteristics of matter that can be changed without changing its composition – characteristics that are directly observable • chemical properties are the characteristics that determine how the composition of matter changes as a result of contact with other matter or the influence of energy – characteristics that describe the behavior of Tro, Chemistry: A 19 matter Molecular Approach

Properties of Matter • Physical Properties – attributes of a substance, does not involve a chemical reaction – color – Temperature – mass – boiling point – melting point - density - volume

Properties of Matter • Chemical properties – how a substance behaves around other substances, involves a chemical reaction – flammability – Reactivity - corrosion

Measurement In Science

All measurements contain the following • A number • A unit following the number indicating the type of measurement made e. g. 12. 0 ft or 100 C

How do scientists report numbers? Scientific notation (book calls it exponential notation) • Used to express very large or very small numbers • Based on powers of ten

How wide is our universe? 210, 000, 000, 000 miles (22 zeros) This number is written in decimal notation. When numbers get this large, it is easier to write them in scientific notation.

Scientific Notation A number is expressed in scientific notation when it is in the form a x 10 n where a is between 1 and 10 and n is an integer

Write the width of the universe in scientific notation. 210, 000, 000, 000 miles Where is the decimal point now? After the last zero. Where would you put the decimal to make this number be between 1 and 10? Between the 2 and the 1

2. 10, 000, 000, 000. How many decimal places did you move the decimal? 23 When the original number is more than 1, the exponent is positive. The answer in scientific notation is 2. 1 x 1023

1) Express 0. 0000000902 in scientific notation. Where would the decimal go to make the number be between 1 and 10? 9. 02 The decimal was moved how many places? 8 When the original number is less than 1, the exponent is negative. 9. 02 x 10 -8

1) Express 0. 0000000902 in scientific notation. Where would the decimal go to make the number be between 1 and 10? 9. 02 The decimal was moved how many places? 8 When the original number is less than 1, the exponent is negative. 9. 02 x 10 -8

Write 28750. 9 in scientific notation. 1. 2. 3. 4. 2. 87509 x 10 -5 2. 87509 x 10 -4 2. 87509 x 105

2) Express 1. 8 x 10 -4 in decimal notation. 0. 00018 3) Express 4. 58 x 106 in decimal notation. 4, 580, 000 On the graphing calculator, scientific notation is done with the button. 4. 58 x 106 is typed 4. 58 6

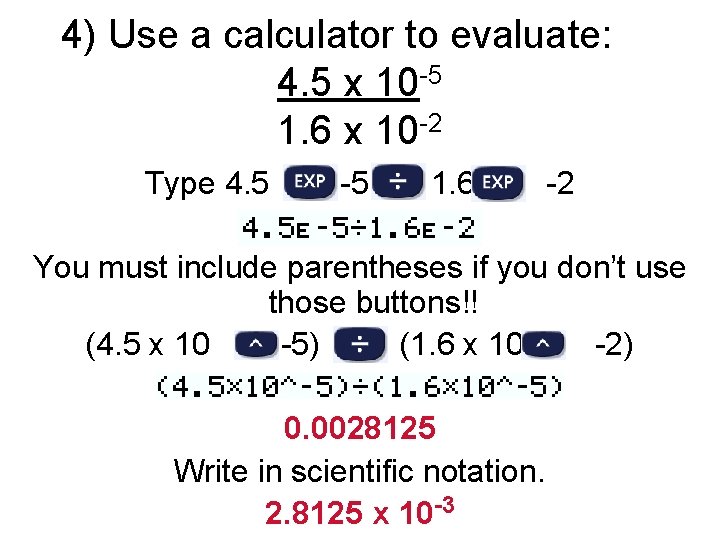
4) Use a calculator to evaluate: 4. 5 x 10 -5 1. 6 x 10 -2 Type 4. 5 -5 1. 6 -2 You must include parentheses if you don’t use those buttons!! (4. 5 x 10 -5) (1. 6 x 10 -2) 0. 0028125 Write in scientific notation. 2. 8125 x 10 -3

5) Use a calculator to evaluate: 7. 2 x 10 -9 2 1. 2 x 10 On the calculator, the answer is: 6. E -11 The answer in scientific notation is 6 x 10 -11 The answer in decimal notation is 0. 000006

6) Use a calculator to evaluate (0. 0042)(330, 000). On the calculator, the answer is 1386. The answer in decimal notation is 1386 The answer in scientific notation is 1. 386 x 103

7) Use a calculator to evaluate (3, 600, 000)(23). On the calculator, the answer is: 8. 28 E +10 The answer in scientific notation is 8. 28 x 10 10 The answer in decimal notation is 82, 800, 000

Write (2. 8 x 103)(5. 1 x 10 -7) in scientific notation. 1. 2. 3. 4. 14. 28 x 10 -4 1. 428 x 10 -3 14. 28 x 1010 1. 428 x 1011

Write in PROPER scientific notation. (Notice the number is not between 1 and 10) 9 8)2. 346 234. 6 x 1011 9) 0. 0642 x 104 on calculator: 642 6. 42 x 10 2

Write 531. 42 x 105 in scientific notation. 1. 2. 3. 4. 5. 6. 7. . 53142 x 102 5. 3142 x 103 53. 142 x 104 531. 42 x 105 53. 142 x 106 5. 3142 x 107. 53142 x 108

Measurement and Significant Figures

What Is a Measurement? • quantitative observation • comparison to an agreed- upon standard • every measurement has a number and a unit Tro, Chemistry: A Molecular Approach 41

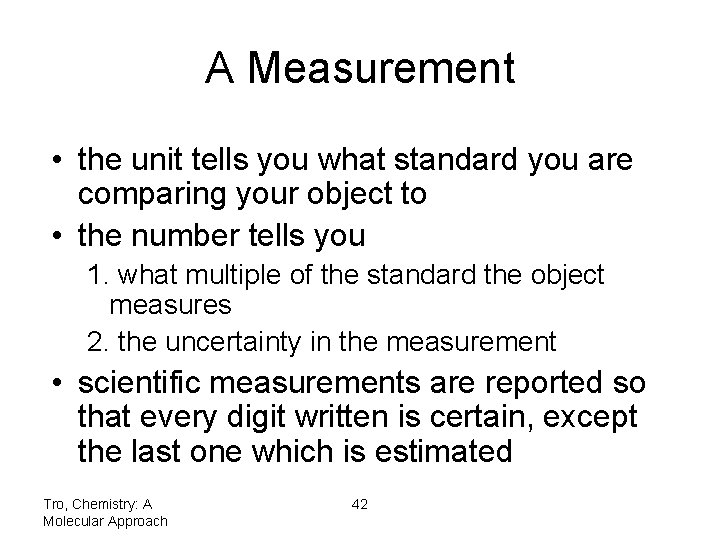
A Measurement • the unit tells you what standard you are comparing your object to • the number tells you 1. what multiple of the standard the object measures 2. the uncertainty in the measurement • scientific measurements are reported so that every digit written is certain, except the last one which is estimated Tro, Chemistry: A Molecular Approach 42

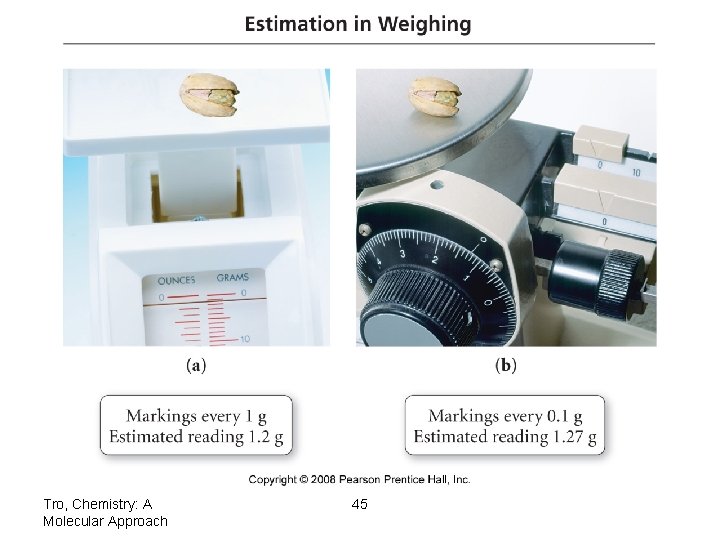
Estimating the Last Digit • for instruments marked with a scale, you get the last digit by estimating between the marks – if possible • mentally divide the space into 10 equal spaces, then estimate how many spaces over the indicator mark is Tro, Chemistry: A Molecular Approach 43

Significant Figures Some numbers are exact: There are 60 seconds in 1 minute 25 cents in 1 quarter 12 eggs in one dozen There is no uncertainty in any of these numbers. In other words there are 12. 00000000000000000 eggs in 1 dozen (add as many zeros as you like)

Tro, Chemistry: A Molecular Approach 45

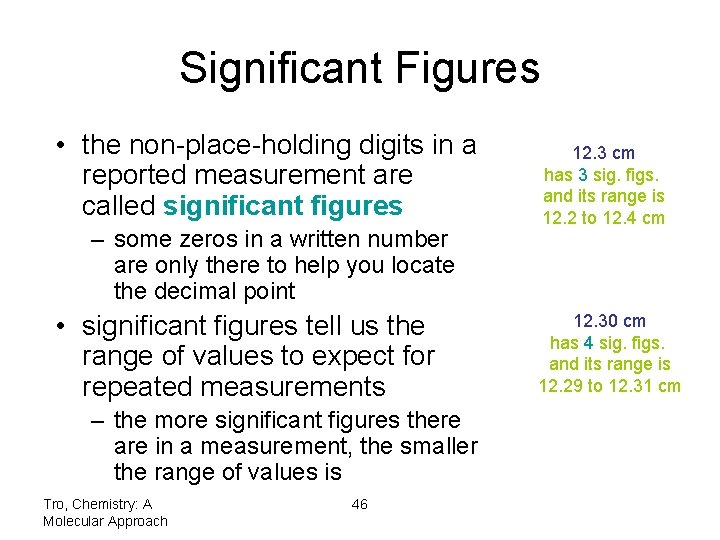
Significant Figures • the non-place-holding digits in a reported measurement are called significant figures – some zeros in a written number are only there to help you locate the decimal point • significant figures tell us the range of values to expect for repeated measurements – the more significant figures there are in a measurement, the smaller the range of values is Tro, Chemistry: A Molecular Approach 46 12. 3 cm has 3 sig. figs. and its range is 12. 2 to 12. 4 cm 12. 30 cm has 4 sig. figs. and its range is 12. 29 to 12. 31 cm

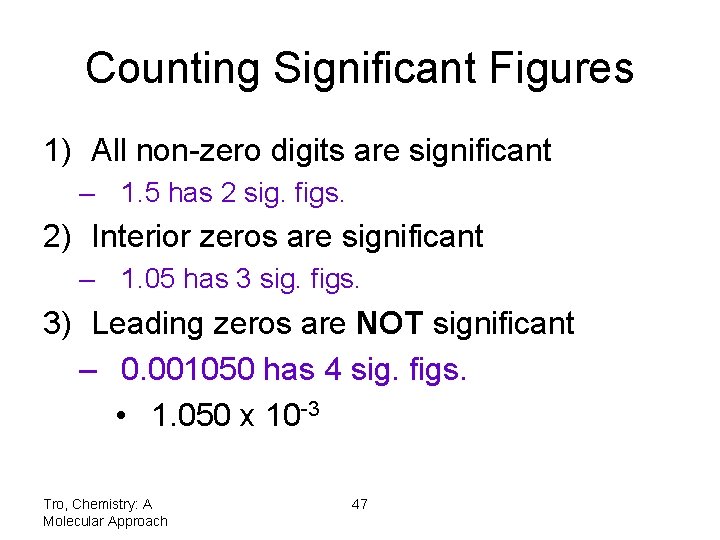
Counting Significant Figures 1) All non-zero digits are significant – 1. 5 has 2 sig. figs. 2) Interior zeros are significant – 1. 05 has 3 sig. figs. 3) Leading zeros are NOT significant – 0. 001050 has 4 sig. figs. • 1. 050 x 10 -3 Tro, Chemistry: A Molecular Approach 47

Counting Significant Figures 4) Trailing zeros may or may not be significant 1) Trailing zeros after a decimal point are significant • 1. 050 has 4 sig. figs. 2) Zeros at the end of a number without a written decimal point are ambiguous and should be avoided by using scientific notation • if 150 has 2 sig. figs. then 1. 5 x 102 • but if 150 has 3 sig. figs. then 1. 50 x 102 Tro, Chemistry: A Molecular Approach 48

Significant Figures and Exact Numbers • Exact numbers have an unlimited number of significant figures • A number whose value is known with complete certainty is exact – from counting individual objects – from definitions • 1 cm is exactly equal to 0. 01 m – from integer values in equations • in the equation for the radius of a circle, the 2 is exact diameter of a circle radius of a circle = Tro, Chemistry: A Molecular Approach 49 2

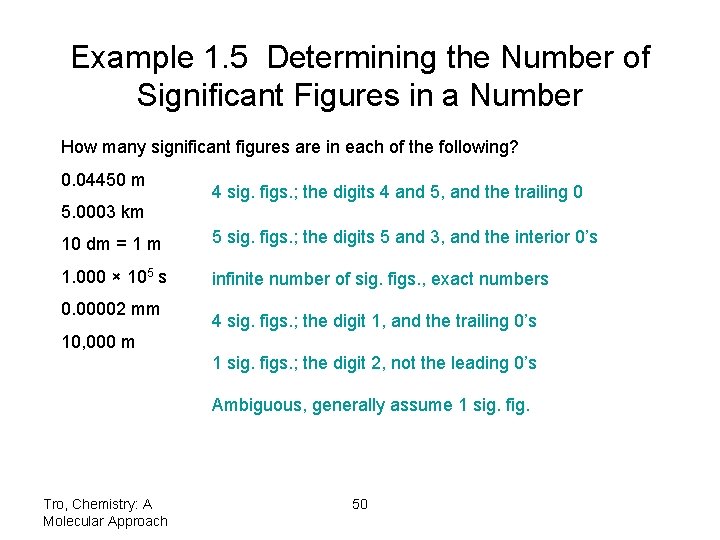
Example 1. 5 Determining the Number of Significant Figures in a Number How many significant figures are in each of the following? 0. 04450 m 5. 0003 km 4 sig. figs. ; the digits 4 and 5, and the trailing 0 10 dm = 1 m 5 sig. figs. ; the digits 5 and 3, and the interior 0’s 1. 000 × 105 s infinite number of sig. figs. , exact numbers 0. 00002 mm 4 sig. figs. ; the digit 1, and the trailing 0’s 10, 000 m 1 sig. figs. ; the digit 2, not the leading 0’s Ambiguous, generally assume 1 sig. fig. Tro, Chemistry: A Molecular Approach 50

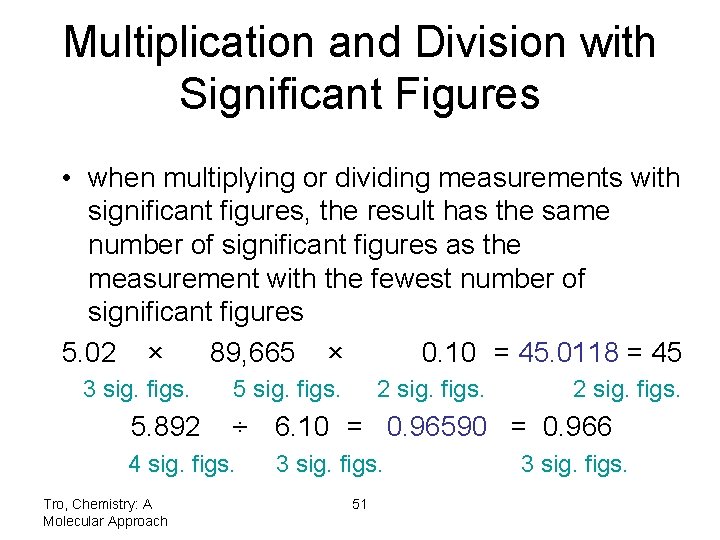
Multiplication and Division with Significant Figures • when multiplying or dividing measurements with significant figures, the result has the same number of significant figures as the measurement with the fewest number of significant figures 5. 02 × 89, 665 × 0. 10 = 45. 0118 = 45 3 sig. figs. 5. 892 5 sig. figs. 2 sig. figs. ÷ 6. 10 = 0. 96590 = 0. 966 4 sig. figs. Tro, Chemistry: A Molecular Approach 2 sig. figs. 3 sig. figs. 51 3 sig. figs.

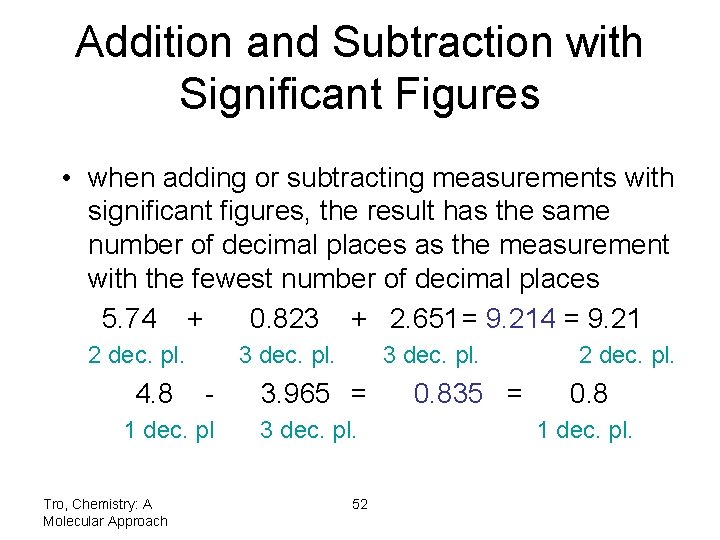
Addition and Subtraction with Significant Figures • when adding or subtracting measurements with significant figures, the result has the same number of decimal places as the measurement with the fewest number of decimal places 5. 74 + 0. 823 + 2. 651= 9. 214 = 9. 21 2 dec. pl. 4. 8 3 dec. pl. - 1 dec. pl Tro, Chemistry: A Molecular Approach 3 dec. pl. 3. 965 = 3 dec. pl. 52 0. 835 = 2 dec. pl. 0. 8 1 dec. pl.

Rounding • when rounding to the correct number of significant figures, if the number after the place of the last significant figure is 1. 0 to 4, round down – drop all digits after the last sig. fig. and leave the last sig. fig. alone – add insignificant zeros to keep the value if necessary 2. 5 to 9, round up – drop all digits after the last sig. fig. and increase the last sig. fig. by one – add insignificant zeros to keep the value if necessary • to avoid accumulating extra error from rounding, round only at the end, keeping track of the last sig. Tro, Chemistry: A 53 Molecular Approach fig. for intermediate calculations

Rounding • rounding to 2 significant figures • 2. 34 rounds to 2. 3 – because the 3 is where the last sig. fig. will be and the number after it is 4 or less • 2. 37 rounds to 2. 4 – because the 3 is where the last sig. fig. will be and the number after it is 5 or greater • 2. 349865 rounds to 2. 3 – because the 3 is where the last sig. fig. will be and the number after it is 4 or less Tro, Chemistry: A Molecular Approach 54

Rounding • rounding to 2 significant figures • 0. 0234 rounds to 0. 023 or 2. 3 × 10 -2 – because the 3 is where the last sig. fig. will be and the number after it is 4 or less • 0. 0237 rounds to 0. 024 or 2. 4 × 10 -2 – because the 3 is where the last sig. fig. will be and the number after it is 5 or greater • 0. 02349865 rounds to 0. 023 or 2. 3 × 10 2 – because the 3 is where the last sig. fig. will be and the number after it is 4 or less Tro, Chemistry: A Molecular Approach 55

Rounding • rounding to 2 significant figures • 234 rounds to 230 or 2. 3 × 102 – because the 3 is where the last sig. fig. will be and the number after it is 4 or less • 237 rounds to 240 or 2. 4 × 102 – because the 3 is where the last sig. fig. will be and the number after it is 5 or greater • 234. 9865 rounds to 230 or 2. 3 × 102 – because the 3 is where the last sig. fig. will be and the number after it is 4 or less Tro, Chemistry: A Molecular Approach 56

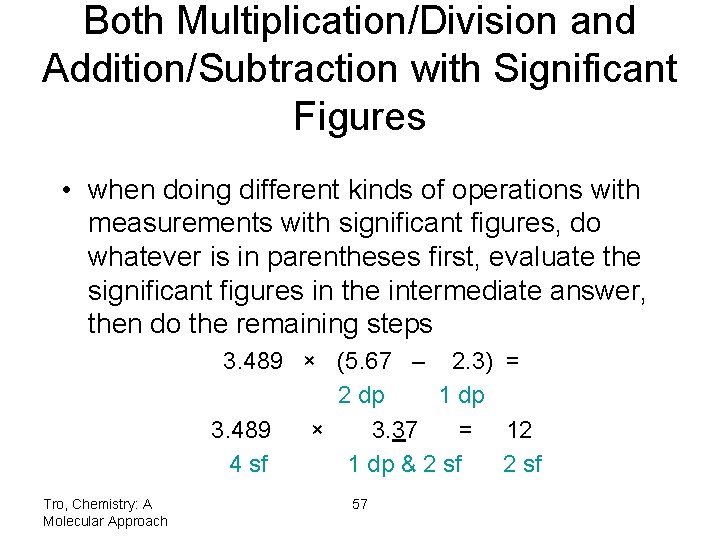
Both Multiplication/Division and Addition/Subtraction with Significant Figures • when doing different kinds of operations with measurements with significant figures, do whatever is in parentheses first, evaluate the significant figures in the intermediate answer, then do the remaining steps 3. 489 × (5. 67 – 2. 3) = 2 dp 1 dp 3. 489 × 3. 37 = 12 4 sf 1 dp & 2 sf Tro, Chemistry: A Molecular Approach 57

Example 1. 6 Perform the following calculations to the correct number of significant figures b) Tro, Chemistry: A Molecular Approach 58

Example 1. 6 Perform the following calculations to the correct number of significant figures b) Tro, Chemistry: A Molecular Approach 59

How many significant figures are there in each of the following? 12. 78 g 3. 16 cm 4 sign. figures 3. 905 mg 4 sign. figures 2. 50 ml 3 sign. figures . 025 ng 2 sign. figures 25000 kg 2. 50 x 104 kg 2, 3, 4, 5? 3 sign. figures

Atlantic-Pacific Rule

Standard Units of Measurement

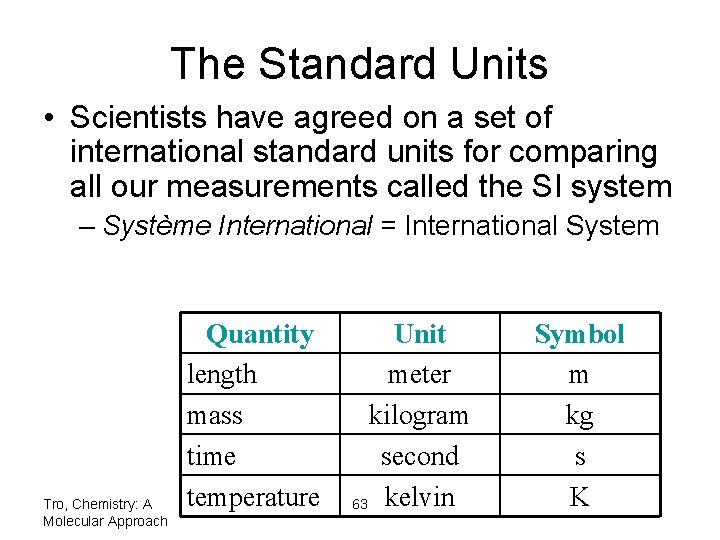
The Standard Units • Scientists have agreed on a set of international standard units for comparing all our measurements called the SI system – Système International = International System Tro, Chemistry: A Molecular Approach Quantity length mass time temperature Unit meter kilogram second 63 kelvin Symbol m kg s K

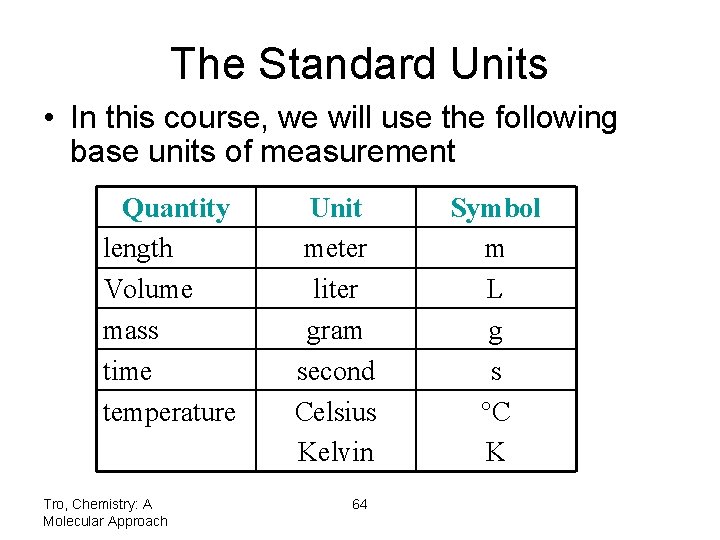
The Standard Units • In this course, we will use the following base units of measurement Quantity length Volume mass time temperature Tro, Chemistry: A Molecular Approach Unit meter liter gram second Celsius Kelvin 64 Symbol m L g s C K

Length • Measure of the two-dimensional distance an object covers – often need to measure lengths that are very long (distances between stars) or very short (distances between atoms) • SI unit = meter – About 3. 37 inches longer than a yard • 1 meter = one ten-millionth the distance from the North Pole to the Equator = distance between marks on standard metal rod = distance traveled by light in a specific period of time • Commonly use centimeters (cm) – 1 m = 100 cm – 1 cm = 0. 01 m = 10 mm – 1 inch = 2. 54 cm (exactly) Tro, Chemistry: A Molecular Approach 65

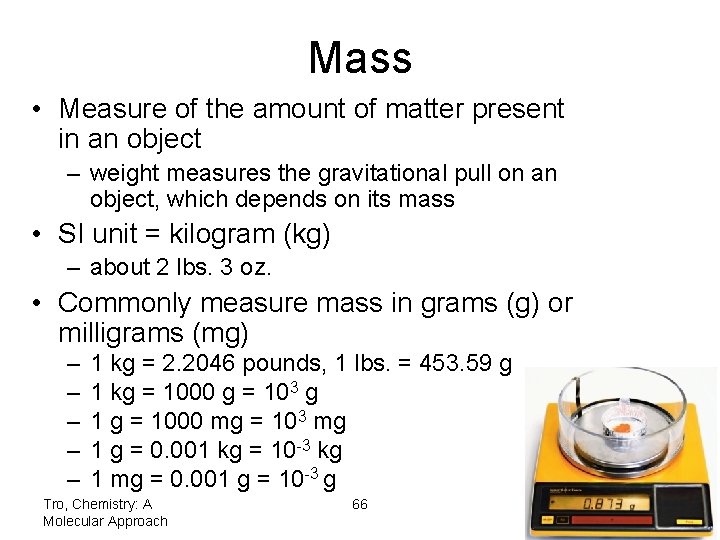
Mass • Measure of the amount of matter present in an object – weight measures the gravitational pull on an object, which depends on its mass • SI unit = kilogram (kg) – about 2 lbs. 3 oz. • Commonly measure mass in grams (g) or milligrams (mg) – – – 1 kg = 2. 2046 pounds, 1 lbs. = 453. 59 g 1 kg = 1000 g = 103 g 1 g = 1000 mg = 103 mg 1 g = 0. 001 kg = 10 -3 kg 1 mg = 0. 001 g = 10 -3 g Tro, Chemistry: A Molecular Approach 66

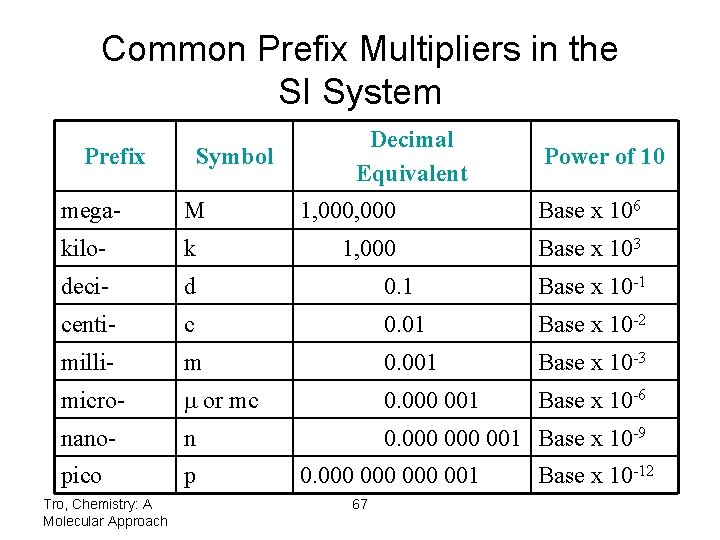
Common Prefix Multipliers in the SI System Prefix Symbol Decimal Equivalent Power of 10 mega- M kilo- k deci- d 0. 1 Base x 10 -1 centi- c 0. 01 Base x 10 -2 milli- m 0. 001 Base x 10 -3 micro- m or mc 0. 000 001 Base x 10 -6 nano- n 0. 000 001 Base x 10 -9 pico p Tro, Chemistry: A Molecular Approach 1, 000 Base x 106 1, 000 Base x 103 0. 000 000 001 67 Base x 10 -12

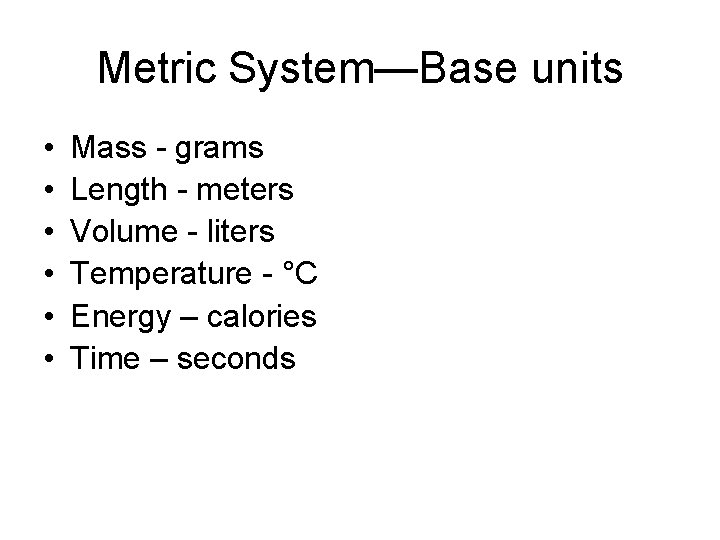
Metric System—Base units • • • Mass - grams Length - meters Volume - liters Temperature - °C Energy – calories Time – seconds

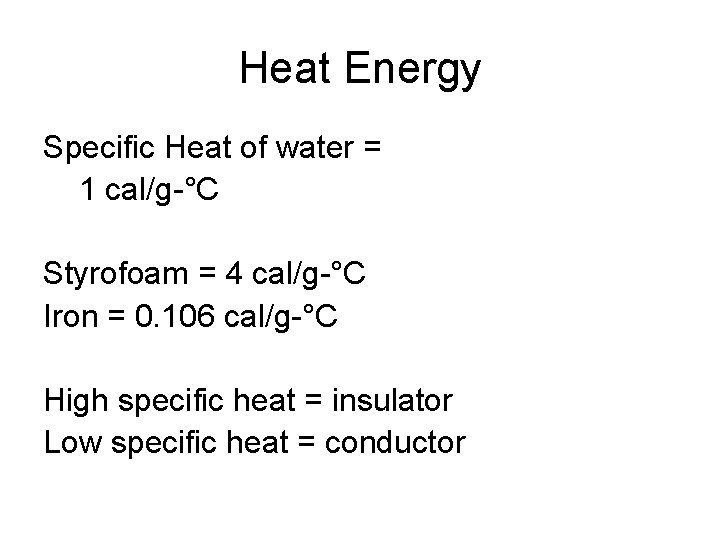
Heat Energy • calorie – amount of heat energy needed to raise the temperature of one gram of water one degree Celsius • Specific Heat – amount of heat energy needed to raise the temperature of one gram of any substance one degree Celsius

Heat Energy Specific Heat of water = 1 cal/g-°C Styrofoam = 4 cal/g-°C Iron = 0. 106 cal/g-°C High specific heat = insulator Low specific heat = conductor

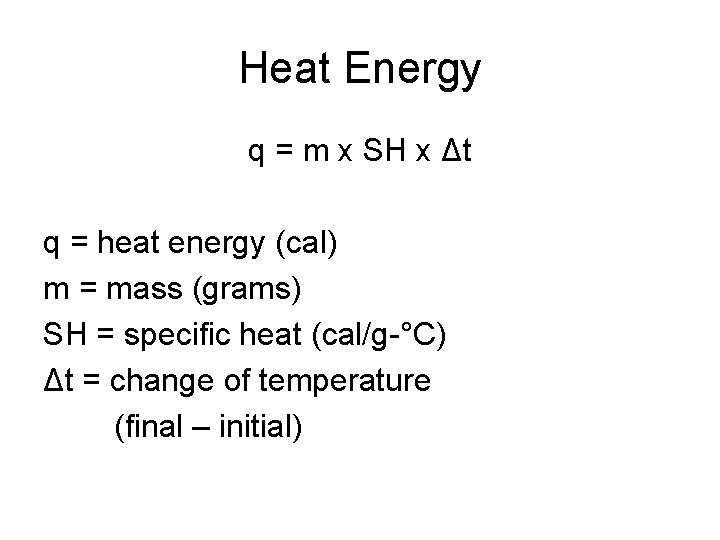
Heat Energy q = m x SH x Δt q = heat energy (cal) m = mass (grams) SH = specific heat (cal/g-°C) Δt = change of temperature (final – initial)

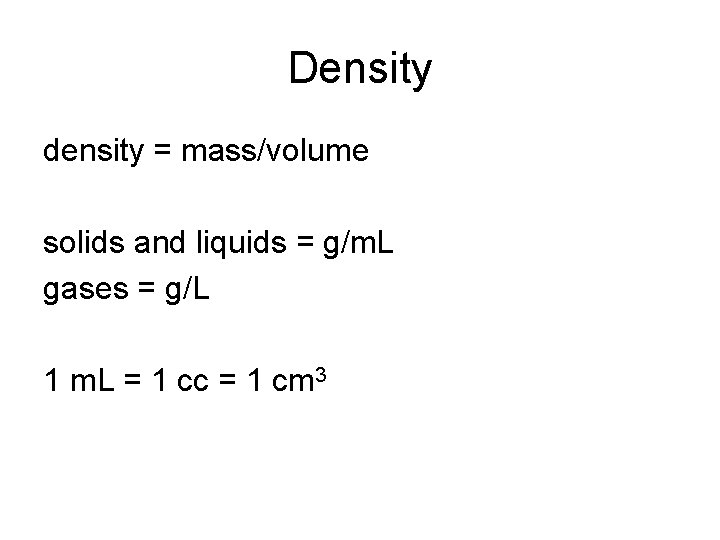
Density

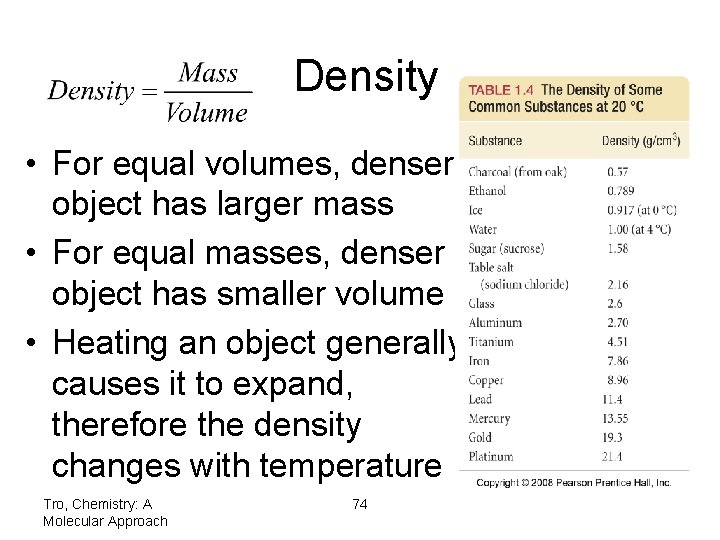
Density density = mass/volume solids and liquids = g/m. L gases = g/L 1 m. L = 1 cc = 1 cm 3

Density • For equal volumes, denser object has larger mass • For equal masses, denser object has smaller volume • Heating an object generally causes it to expand, therefore the density changes with temperature Tro, Chemistry: A Molecular Approach 74

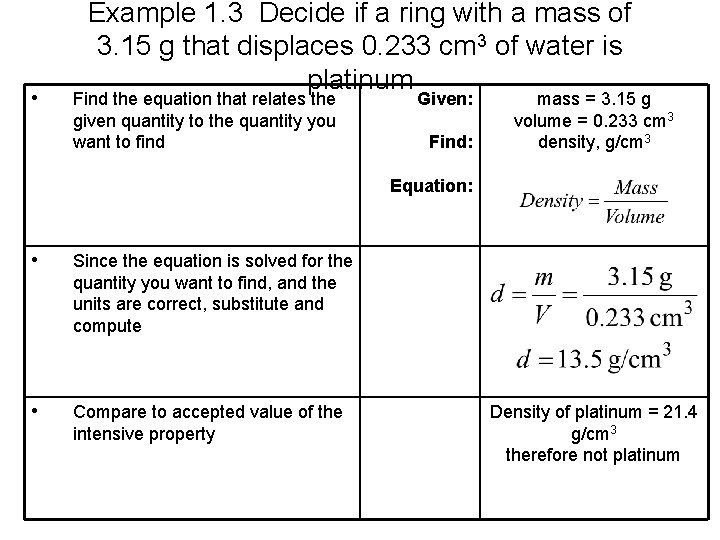
• Example 1. 3 Decide if a ring with a mass of 3. 15 g that displaces 0. 233 cm 3 of water is platinum Find the equation that relates the given quantity to the quantity you want to find Given: Find: mass = 3. 15 g volume = 0. 233 cm 3 density, g/cm 3 Equation: • Since the equation is solved for the quantity you want to find, and the units are correct, substitute and compute • Compare to accepted value of the intensive property Density of platinum = 21. 4 g/cm 3 therefore not platinum
 Matter energy and measurement
Matter energy and measurement Energy naturally flows from warmer matter to cooler matter.
Energy naturally flows from warmer matter to cooler matter. Chemistry chapter 3 scientific measurement
Chemistry chapter 3 scientific measurement Chapter 3 scientific measurement
Chapter 3 scientific measurement Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Lesson 1 understanding science answer key
Lesson 1 understanding science answer key Lesson 1 understanding science answer key
Lesson 1 understanding science answer key Understanding science lesson 1 answer key
Understanding science lesson 1 answer key Gray matter in the brain
Gray matter in the brain Cerebral aqueduct
Cerebral aqueduct Gray matter and white matter
Gray matter and white matter What does grey matter do
What does grey matter do Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis The mole a measurement of matter answer key
The mole a measurement of matter answer key 10.1 the mole a measurement of matter
10.1 the mole a measurement of matter Density is a qualitative measurement of matter
Density is a qualitative measurement of matter The mole a measurement of matter
The mole a measurement of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter What is scientific matter
What is scientific matter Scientific inquiry vs scientific method
Scientific inquiry vs scientific method How is a scientific law different from a scientific theory?
How is a scientific law different from a scientific theory? Phosphorus cycle
Phosphorus cycle Section 16.1 thermal energy and matter
Section 16.1 thermal energy and matter Matter and thermal energy section 1
Matter and thermal energy section 1 Science matter and energy
Science matter and energy Dark matter and dark energy presentation
Dark matter and dark energy presentation Unit 2 matter and energy
Unit 2 matter and energy Tropical levels
Tropical levels Lesson outline lesson 1 solids liquids and gases answer key
Lesson outline lesson 1 solids liquids and gases answer key Mind map on states of matter
Mind map on states of matter Whats the study of matter and energy
Whats the study of matter and energy Energy that travels through
Energy that travels through Properties of waves
Properties of waves Labeled energy pyramid
Labeled energy pyramid What does temperature measure
What does temperature measure Chapter 7 energy conservation of energy
Chapter 7 energy conservation of energy Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton là gì
Tư thế worm breton là gì Chúa yêu trần thế
Chúa yêu trần thế Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên?
Thế nào là giọng cùng tên? Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000 Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì