Matter and Energy Matter and energy interact and

























![REGENTS QUESTION: Determine the boiling point of the sample at standard pressure. [1] 95 REGENTS QUESTION: Determine the boiling point of the sample at standard pressure. [1] 95](https://slidetodoc.com/presentation_image_h2/c5d6bcaa58cc604b7a6fdbe6081dc5fe/image-44.jpg)












- Slides: 65

Matter and Energy Matter and energy interact and cause changes in matter. 1

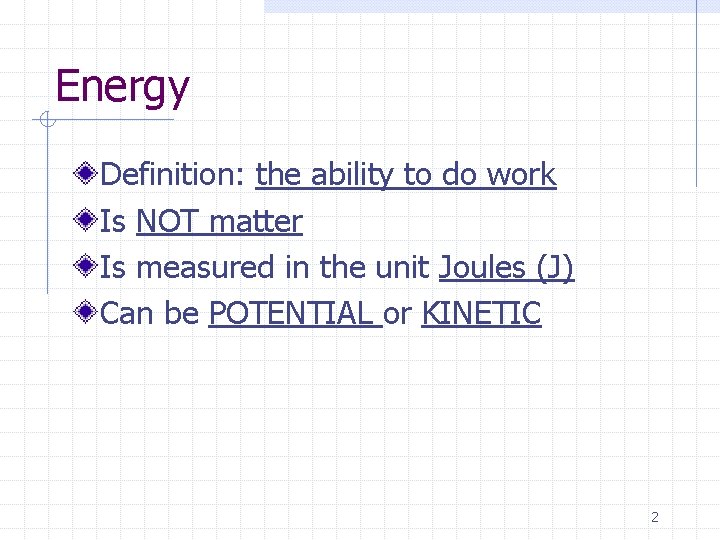
Energy Definition: the ability to do work Is NOT matter Is measured in the unit Joules (J) Can be POTENTIAL or KINETIC 2

Potential Energy that is stored (i. e. in a chemical bond). Something has the “potential” to do some kind of work Example: the child at the top of the slide has potential energy 3

REGENTS QUESTION: Given the balanced equation: F 2 + energy → F + F Which statement describes what occurs during this reaction? (1) Energy is absorbed as a bond is formed. (2) Energy is absorbed as a bond is broken. (3) Energy is released as a bond is formed. (4) Energy is released as a bond is broken. 4

Kinetic Energy of motion Example: the child going down the slide now has kinetic energy 5

6

REGENTS QUESTION: The graph below represents the relationship between time and temperature as heat is added at a constant rate to a sample of a substance. During interval AB, which energy change occurs for the particles in this sample? (1)The potential energy of the particles increases. (2) The potential energy of the particles decreases. (3) The average kinetic energy of the particles increases. (4) The average kinetic energy of the particles decreases. 7

So why do we want to study energy? Energy = the ability to do work! Having something do work is great! Examples: our bodies need energy to do work, our cars rely on energy to do work, our homes need to be heated via energy, etc… 8

Different types of energy… Light is waves, visible or invisible Electrical involves moving electrons Heat movement of molecules Chemical is contained in foods Nuclear responsible for the sun Sound waves/vibrations of molecules Mechanical involves moving objects Magnetic opposing poles 9

REGENTS QUESTION: Three forms of energy are (1) chemical, exothermic, and temperature (2) chemical, thermal, and electromagnetic (3) electrical, nuclear, and temperature (4) electrical, mechanical, and endothermic 10

Light Responsible for colors Responsible for sight We have found ways to use light to improve how much we can see and what we see (example: TV) 11

Chemical and Electrical Energy Energizes everything from remote controls to cars. 12

Chemical Energy Chemical energy from crude oil (natural, nonrenewable resources) is used to heat our homes and run factories that produce consumer goods. 13

Law of Conservation of Energy, like matter, is neither created nor destroyed, rather it is converted. 14

Examples of Conservation of Energy When you watch TV, it starts as electrical energy and converts to radiant and sound energy. The radiant energy (or light energy) goes into your eye and converts to electrical energy in your nerves and then to the brain. The sound energy (vibrations) go to your ear drum where is vibrates sending electrical impulses to the brain. 15

REGENTS QUESTION: Which quantities must be conserved in all chemical reactions? (1) mass, charge, density (2) mass, charge, energy (3) charge, volume, density (4) charge, volume, energy 16

REGENTS QUESTION Which quantities must be conserved in all chemical reactions? (1) mass, charge, density (2) mass, charge, energy (3) charge, volume, density (4) charge, volume, energy 17

Summary: What will we study in this unit? What is heat? How is it different from temperature? How does energy relate to chemical reactions? How energy relates to phase changes? 18

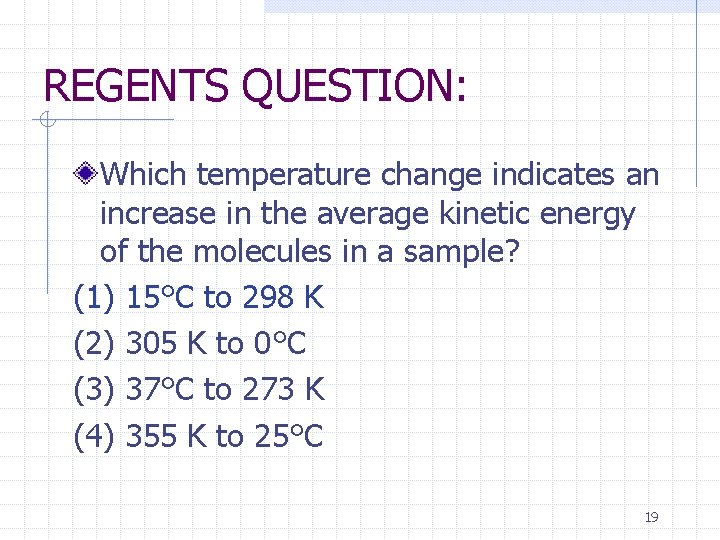
REGENTS QUESTION: Which temperature change indicates an increase in the average kinetic energy of the molecules in a sample? (1) 15°C to 298 K (2) 305 K to 0°C (3) 37°C to 273 K (4) 355 K to 25°C 19

REGENTS QUESTION: Which temperature change indicates an increase in the average kinetic energy of the molecules in a sample? (1) 15°C to 298 K (2) 305 K to 0°C (3) 37°C to 273 K (4) 355 K to 25°C 20

Understanding Heat Flow Heat (q) is defined as the energy that transfers from one object to another. Heat flows from warm cool. What will happen if the two objects are touching? (example) 21

REGENTS QUESTION: A student made a copper bracelet by hammering a small copper bar into the desired shape. The bracelet has a mass of 30. 1 grams and was at a temperature of 21°C in the classroom. After the student wore the bracelet, the bracelet reached a temperature of 33°C. Later, the student removed the bracelet and placed it on a desk at home, where it cooled from 33°C to 19°C. Explain, in terms of heat flow, the change in the temperature of the bracelet when the student wore the bracelet. [1] 22

REGENTS QUESTION A student made a copper bracelet by hammering a small copper bar into the desired shape. The bracelet has a mass of 30. 1 grams and was at a temperature of 21°C in the classroom. After the student wore the bracelet, the bracelet reached a temperature of 33°C. Later, the student removed the bracelet and placed it on a desk at home, where it cooled from 33°C to 19°C. Explain, in terms of heat flow, the change in the temperature of the bracelet when the student wore the bracelet. [1] Heat moves from the students hand into the bracelet causing the temperature of the bracelet to increase. 23

Heat Energy vs. Temperature is measure of the heat flow. Temperature is a measure of the average kinetic energy of the particles in matter. 24

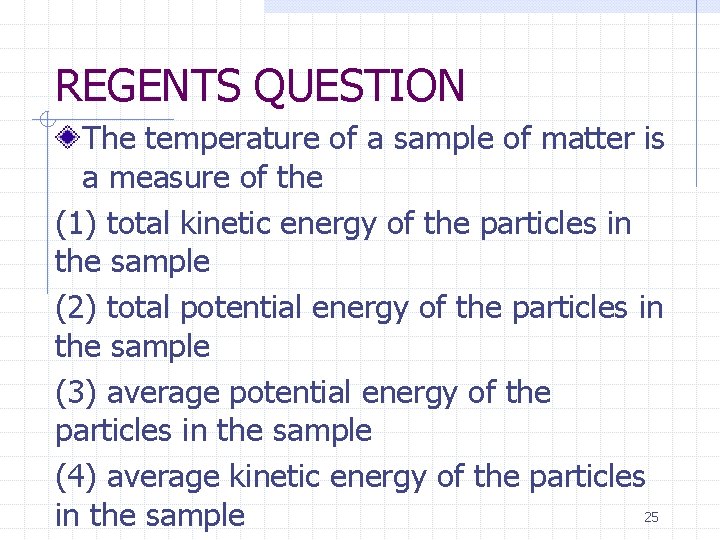
REGENTS QUESTION The temperature of a sample of matter is a measure of the (1) total kinetic energy of the particles in the sample (2) total potential energy of the particles in the sample (3) average potential energy of the particles in the sample (4) average kinetic energy of the particles 25 in the sample

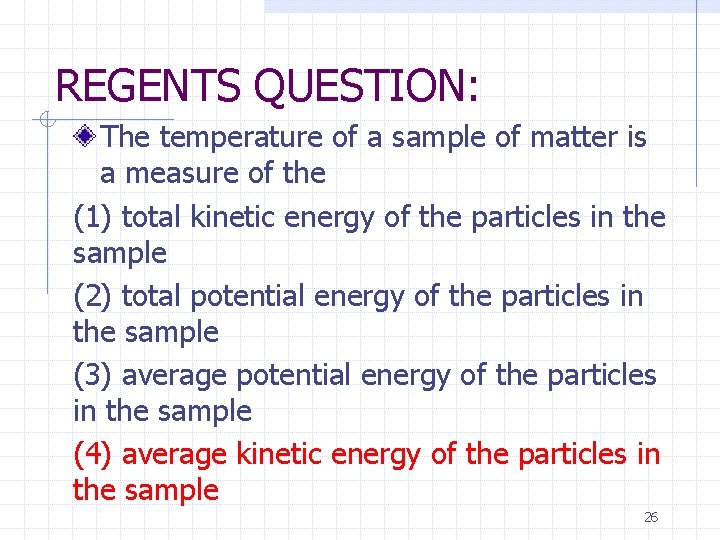
REGENTS QUESTION: The temperature of a sample of matter is a measure of the (1) total kinetic energy of the particles in the sample (2) total potential energy of the particles in the sample (3) average potential energy of the particles in the sample (4) average kinetic energy of the particles in the sample 26

Heat Energy and Changes in Matter In virtually all changes in matter, energy is released or absorbed. System vs. Surroundings (together they make the universe). 27

Examples 28

REGENTS QUESTION: A few pieces of dry ice, CO 2(s), at – 78°C are placed in a flask that contains air at 21°C. The flask is sealed by placing an uninflated balloon over the mouth of the flask. As the balloon inflates, the dry ice disappears and no liquid is observed in the flask. State the direction of heat flow that occurs between the dry ice and the air in the flask. [1] Write the name of the process that occurs as the dry ice undergoes a phase change in the flask. [1] 29

Exothermic Reactions (Changes) Exothermic reactions RELEASE ENERGY (i. e. explosions). A good way to remember this is to associate “EXO” with “OUT”. Has a –q value because heat is leaving the system. Heat is a product. 30

Endothermic Reactions (Changes) Endothermic reactions ABSORB ENERGY A good way to remember this is to associate “ENDO” with “INSIDE”. Has a +q value because heating is entering the system. Heat is a reactant. 31

REGENTS QUESTION What occurs when two fluorine atoms react to produce a fluorine molecule? (1) Energy is absorbed as a bond is broken. (2) Energy is absorbed as a bond is formed. (3) Energy is released as a bond is broken. (4) Energy is released as a bond is formed. 32

REGENTS QUESTION: What occurs when two fluorine atoms react to produce a fluorine molecule? (1) Energy is absorbed as a bond is broken. (2) Energy is absorbed as a bond is formed. (3) Energy is released as a bond is broken. (4) Energy is released as a bond is formed. 33

Activation Energy Sometimes reactions can’t occur on their own (they can be exothermic). They need a little input of energy to get it started. This energy is called ACTIVATION ENERGY. Can you think of a common example of a reaction that requires activation energy? 34

Energy and Phase Changes Energy of particles of matter relates to the phase or state of matter (solid, liquid, or gas) Therefore, changes in energy result in changes of matter. Let’s review what we already know… 35

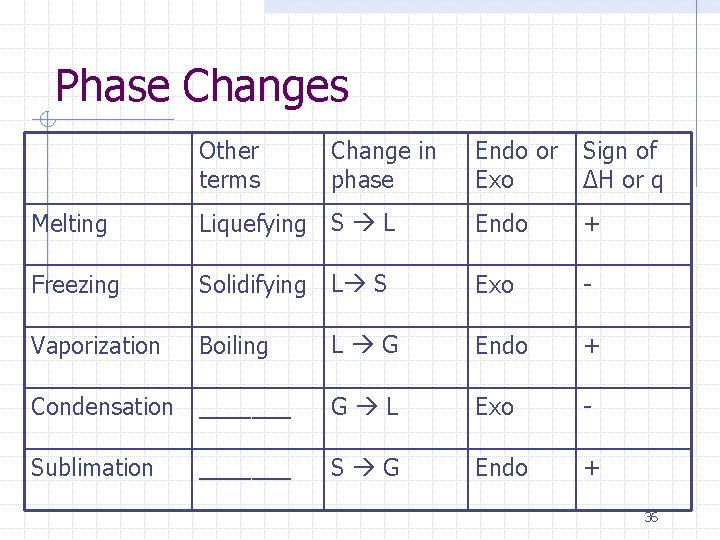
Phase Changes Other terms Change in phase Endo or Exo Sign of ΔH or q Endo + Melting Liquefying S L Freezing Solidifying L S Exo - Vaporization Boiling L G Endo + Condensation _______ G L Exo - Sublimation _______ S G Endo + 36

Monitoring Energy in Phase Changes Have you ever sat and watched a pot or kettle of water boil? Did you ever wonder how energy from the stove causes the water to change phase? Have you ever thought about PE and KE changes in the process of heating water? 37

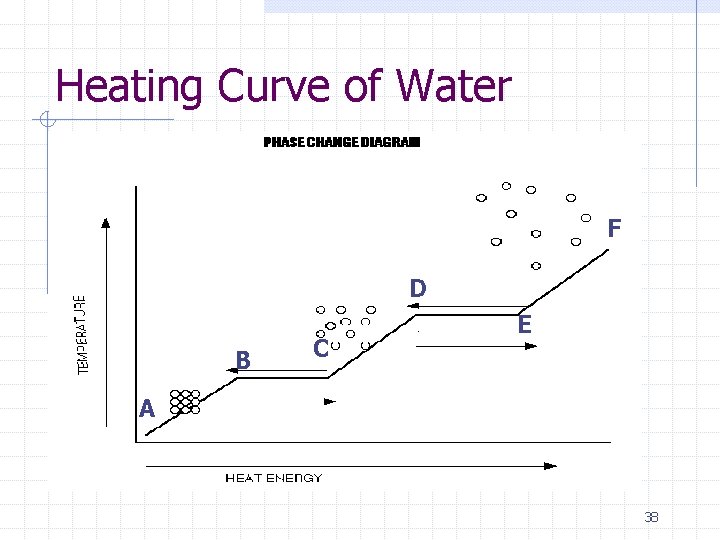
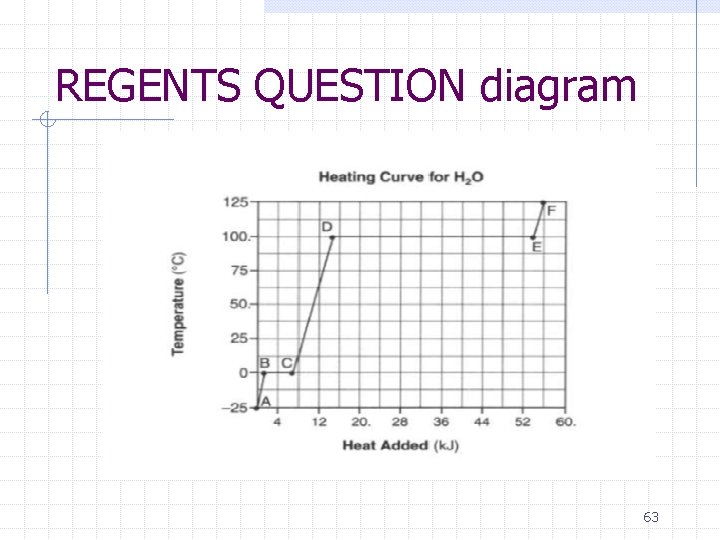
Heating Curve of Water F D B C E A 38

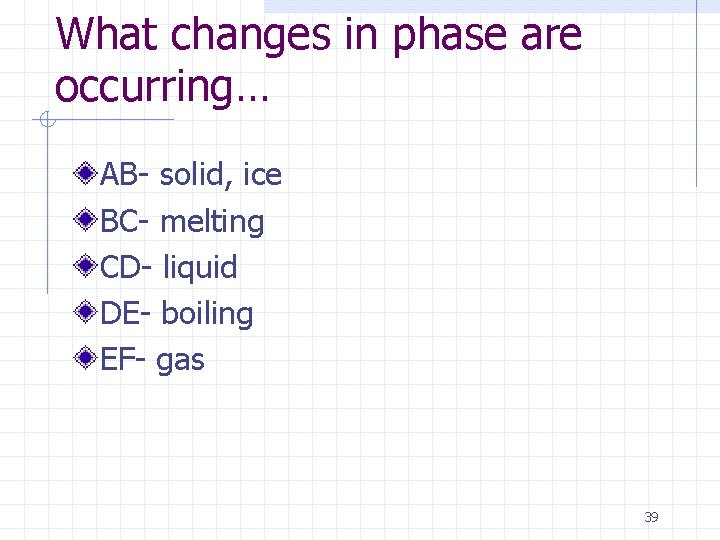
What changes in phase are occurring… AB- solid, ice BC- melting CD- liquid DE- boiling EF- gas 39

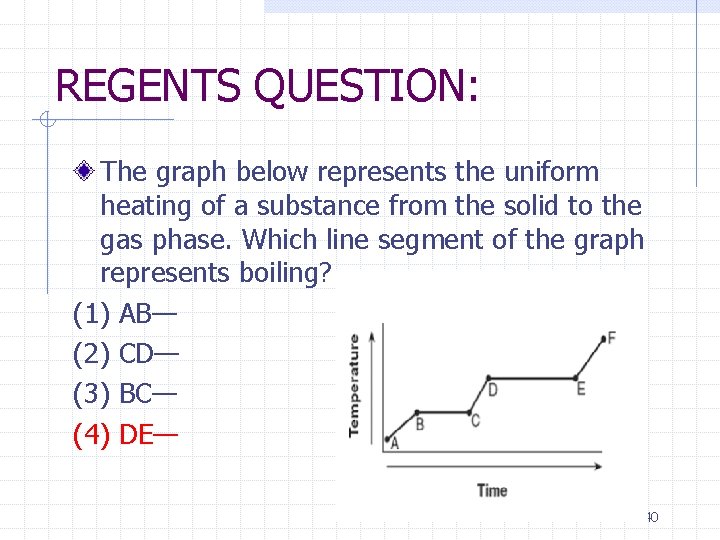
REGENTS QUESTION: The graph below represents the uniform heating of a substance from the solid to the gas phase. Which line segment of the graph represents boiling? (1) AB— (2) CD— (3) BC— (4) DE— 40

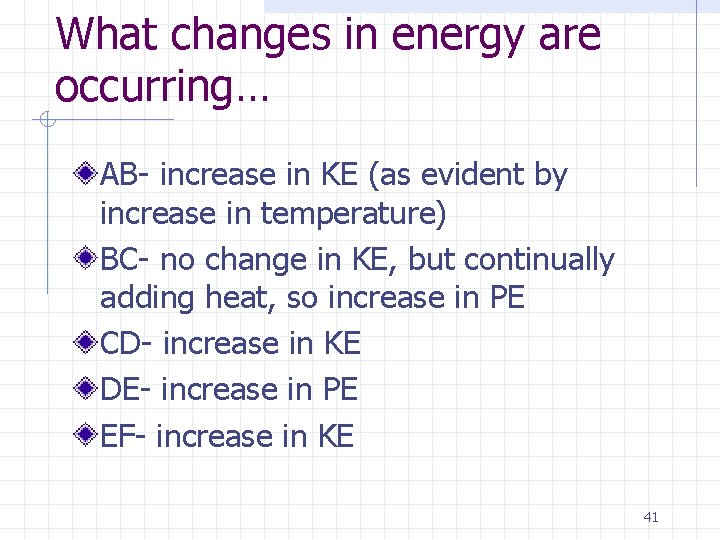
What changes in energy are occurring… AB- increase in KE (as evident by increase in temperature) BC- no change in KE, but continually adding heat, so increase in PE CD- increase in KE DE- increase in PE EF- increase in KE 41

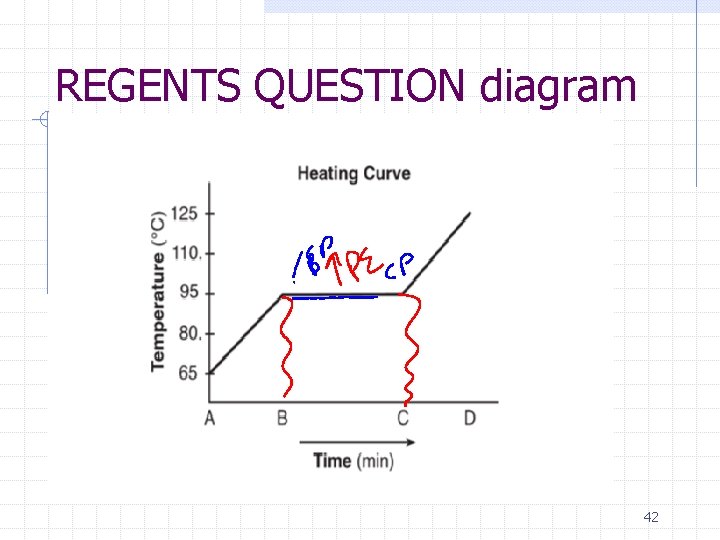
REGENTS QUESTION diagram 42

REGENTS QUESTION: A sample of a substance is a liquid at 65°C. The sample is heated uniformly to 125°C. The heating curve for the sample at standard pressure is shown below. Determine the boiling point of the sample at standard pressure. [1] State what happens to the potential energy of the particles of the sample during time interval BC. [1] 43
![REGENTS QUESTION Determine the boiling point of the sample at standard pressure 1 95 REGENTS QUESTION: Determine the boiling point of the sample at standard pressure. [1] 95](https://slidetodoc.com/presentation_image_h2/c5d6bcaa58cc604b7a6fdbe6081dc5fe/image-44.jpg)
REGENTS QUESTION: Determine the boiling point of the sample at standard pressure. [1] 95 OC State what happens to the potential energy of the particles of the sample during time interval BC. [1] Potential energy increases ( heating curve) 44

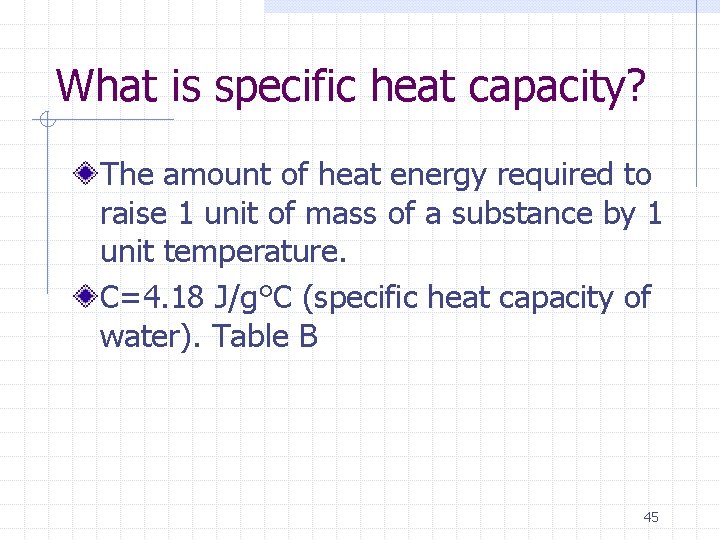
What is specific heat capacity? The amount of heat energy required to raise 1 unit of mass of a substance by 1 unit temperature. C=4. 18 J/g°C (specific heat capacity of water). Table B 45

46

Heat of Fusion Heat of fusion (Hf) = amount of heat energy absorbed or released when melting or freezing. See Reference Table B for values Ex: Hf H 2 O = 334 J/g 47

Heat of Vaporization Heat of vaporization = heat absorbed or released when vaporizing or condensing. See Reference Tables for values. Ex: HVH 2 O = 2260 J/g 48

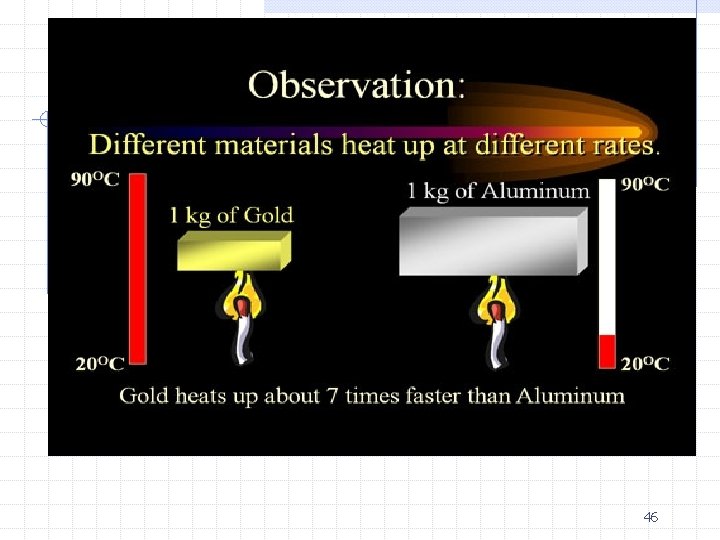
Kinetic Theory of Heat Molecules and atoms are constantly in motion, even in the SOLID phase. They are said to have kinetic energy or the energy of motion. As the energy of the particles increases, temperature increases. 49

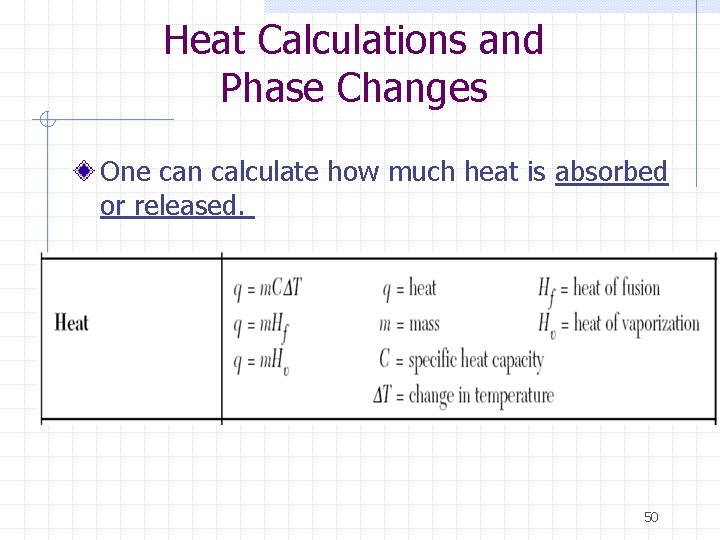
Heat Calculations and Phase Changes One can calculate how much heat is absorbed or released. 50

Potential energy If the problem says… Melt/freeze Vaporize condense At 0°C (melting/freezing point) At 100°C (boiling/condensing point) Use Q = m. Hf (melting/freezing) or Q = m. Hv (vaporization/condensation) 51

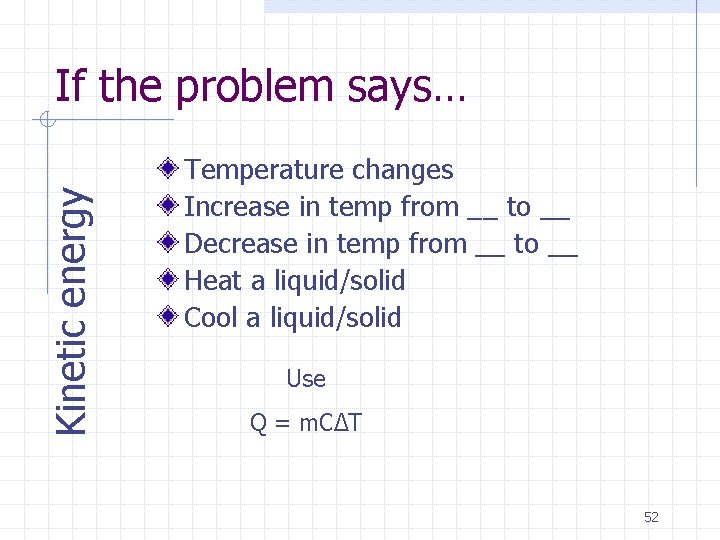
Kinetic energy If the problem says… Temperature changes Increase in temp from __ to __ Decrease in temp from __ to __ Heat a liquid/solid Cool a liquid/solid Use Q = m. CΔT 52

Examples How much heat is needed to melt 10. 5 grams of ice at 0°C? 53

ANSWER Use Q = m. Hf Q = (10. 5 g) (334 J/g) Q= 3507 J 54

Examples What mass of liquid water can be vaporized if 680 J of heat energy is added at 100°C? 55

ANSWER Use Q = m. Hv 680 J = (m) (2260 J/g) m= 0. 30 g 56

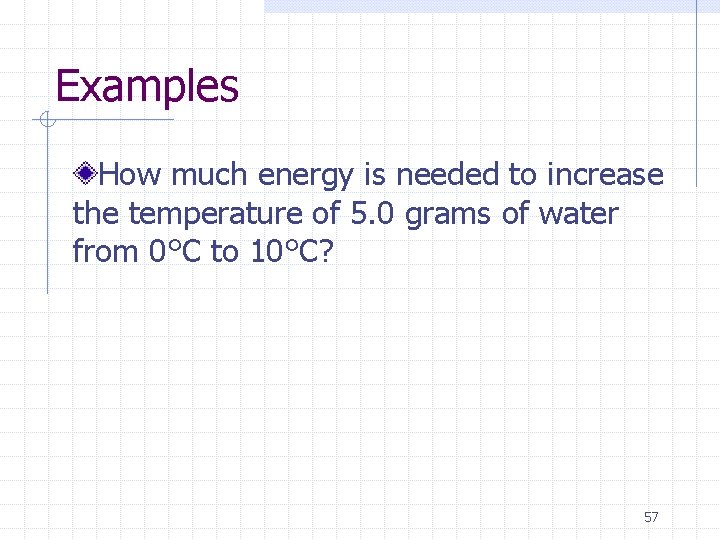
Examples How much energy is needed to increase the temperature of 5. 0 grams of water from 0°C to 10°C? 57

ANSWER Use Q = m. CΔT Q = (5. 0 g) (4. 18 J/g°C)(10 -0) Q= 209 J 58

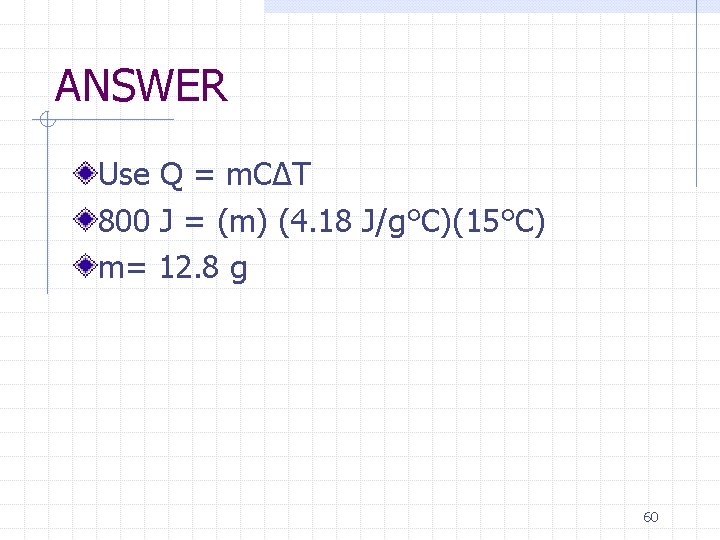
Examples What is the mass of water that can be increased in temperature by 15°C by the addition of 800 J? 59

ANSWER Use Q = m. CΔT 800 J = (m) (4. 18 J/g°C)(15°C) m= 12. 8 g 60

Calorimetry Used to measure amount of heat released or absorbed during a chemical/physical change that occurs in water solution. “calorimeter” is used to measure the change in temperature of water surrounding a reaction. 61

Cheap Calorimeter- insulation 62

REGENTS QUESTION diagram 63

REGENTS QUESTION: Base your answers to questions 1 through 3 on the information below and on your knowledge of chemistry. Starting as a solid at 25°C, a sample of H 2 O is heated at a constant rate until the sample is at 125°C. This heating occurs at standard pressure. The graph below represents the relationship between temperature and heat added to the sample. 1. Describe what happens to both the potential energy and the average kinetic energy of the molecules in the H 2 O sample during interval AB. [1] 2. Using the graph, determine the total amount of heat added to the sample during interval CD. [1] 3. Explain, in terms of heat of fusion and heat of vaporization, why the heat added during interval DE is greater than the heat added during interval BC for this sample of water. [1] 64

ANSWERS 1. Describe what happens to both the potential energy and the average kinetic energy of the molecules in the H 2 O sample during interval AB. [1]Kinetic energy increases while the potential energy remains the same 2. Using the graph, determine the total amount of heat added to the sample during interval CD. [1] 8 k. J +/- 1 3. Explain, in terms of heat of fusion and heat of vaporization, why the heat added during interval DE is greater than the heat added during interval BC for this sample of water. [1] takes longer to boil the substance than to melt it or Hv of water is 2260 J/g and the Hf is 334 J/g 65
 Kesler science sound waves answer key
Kesler science sound waves answer key Flow of energy vs flow of matter
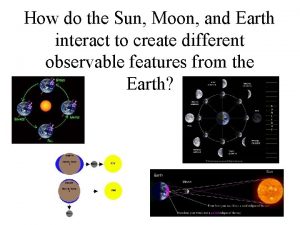
Flow of energy vs flow of matter How do the sun moon and earth interact
How do the sun moon and earth interact Importance of social interaction
Importance of social interaction Revolution moon
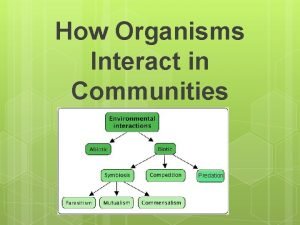
Revolution moon How does biosphere interact with hydrosphere
How does biosphere interact with hydrosphere Member of the same species
Member of the same species Parasitism
Parasitism How does biosphere interact with hydrosphere
How does biosphere interact with hydrosphere How do magnetic poles interact
How do magnetic poles interact What are sphere interactions?
What are sphere interactions? Tectonic plates interact at places called plate
Tectonic plates interact at places called plate Interact club handbook
Interact club handbook Organizing students to interact with new content
Organizing students to interact with new content How do similar (s-s or n-n) magnetic poles interact?
How do similar (s-s or n-n) magnetic poles interact? How does mirabella interact with the rest of the pack
How does mirabella interact with the rest of the pack Rotary club sergeant at arms duties
Rotary club sergeant at arms duties Answer
Answer Sensed time in dance
Sensed time in dance Social interaction in sociology
Social interaction in sociology Interact
Interact Interact
Interact Body interact answers
Body interact answers Interact conference 2018 teachstone
Interact conference 2018 teachstone Interact
Interact Grey matter
Grey matter Gyrus and sulcus function
Gyrus and sulcus function Gray matter and white matter
Gray matter and white matter Matter
Matter Section 1 composition of matter
Section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Composition of matter section 1
Composition of matter section 1 Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Phosphorus cycle
Phosphorus cycle Which reverses the normal flow of thermal energy
Which reverses the normal flow of thermal energy Matter and thermal energy section 1
Matter and thermal energy section 1 Science matter and energy
Science matter and energy Matter energy and measurement
Matter energy and measurement Dark matter and dark energy ppt
Dark matter and dark energy ppt Unit 2 matter and energy
Unit 2 matter and energy Swan food web
Swan food web The science duo physical and chemical changes
The science duo physical and chemical changes Matter concept map
Matter concept map States of matter foldable
States of matter foldable Mechanical and electromagnetic waves similarities
Mechanical and electromagnetic waves similarities Pyramid of energy
Pyramid of energy Wave medium
Wave medium Waves transfer energy without transferring
Waves transfer energy without transferring Thermal energy states of matter
Thermal energy states of matter Rhythmic movement that carries energy through matter
Rhythmic movement that carries energy through matter Oikos meaning
Oikos meaning Waves are repeating disturbances that transfer
Waves are repeating disturbances that transfer Thermal energy vs heat
Thermal energy vs heat Use of heat
Use of heat Internal energy of matter
Internal energy of matter The sun converts matter into energy in what zone
The sun converts matter into energy in what zone A disturbance that transmits energy through matter or space
A disturbance that transmits energy through matter or space What transmits energy without transferring matter
What transmits energy without transferring matter Section 2 describing energy (continued)
Section 2 describing energy (continued) Primary energy and secondary energy
Primary energy and secondary energy Hypdro
Hypdro Helmholtz free energy
Helmholtz free energy Renewable energy and energy efficiency partnership
Renewable energy and energy efficiency partnership Potential energy examples
Potential energy examples