THERMAL ENERGY AND MATTER Ch 16 1 True



- Slides: 28

THERMAL ENERGY AND MATTER Ch 16. 1

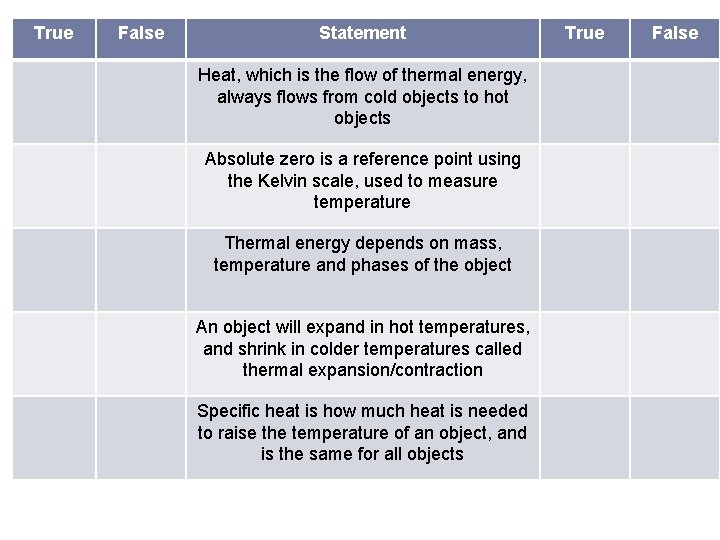
True False Statement Heat, which is the flow of thermal energy, always flows from cold objects to hot objects Absolute zero is a reference point using the Kelvin scale, used to measure temperature Thermal energy depends on mass, temperature and phases of the object An object will expand in hot temperatures, and shrink in colder temperatures called thermal expansion/contraction Specific heat is how much heat is needed to raise the temperature of an object, and is the same for all objects True False

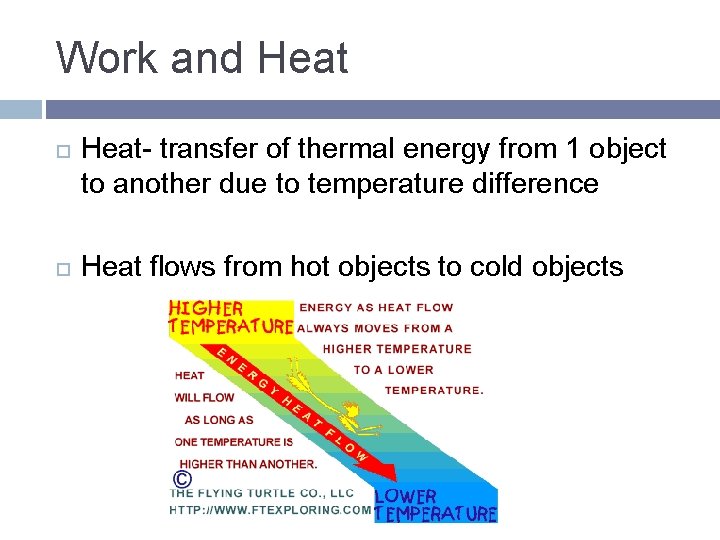
Work and Heat- transfer of thermal energy from 1 object to another due to temperature difference Heat flows from hot objects to cold objects

Temperature Temperaturemeasure of how hot or cold an object is compared to a reference point � °C= boiling/freezing point of water � Absolute zeroreference point in K, which is 0 K Temperature relates to the kinetic energy of particles � hot= faster particles � cold= slower particles

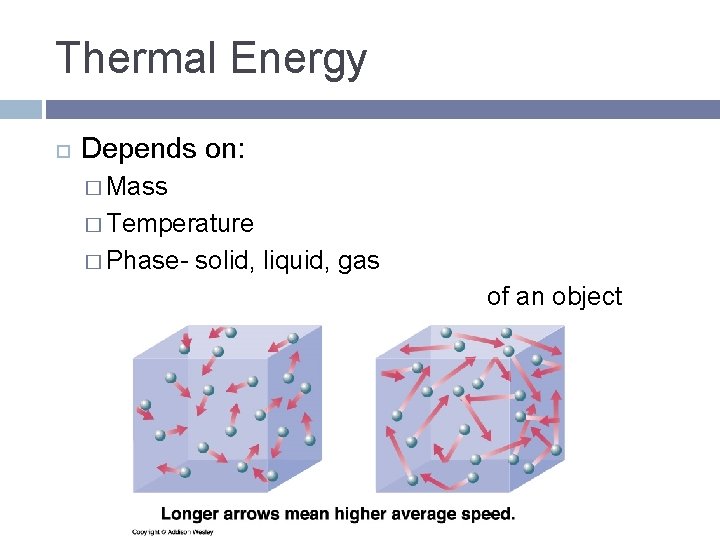
Thermal Energy Depends on: � Mass � Temperature � Phase- solid, liquid, gas of an object

Thermal Contraction and Expansion Thermal Expansion- increase volume due to temperature increase � Particles move farther apart Thermometers � Increase temp. = alcohol moves/expands = temperature you read

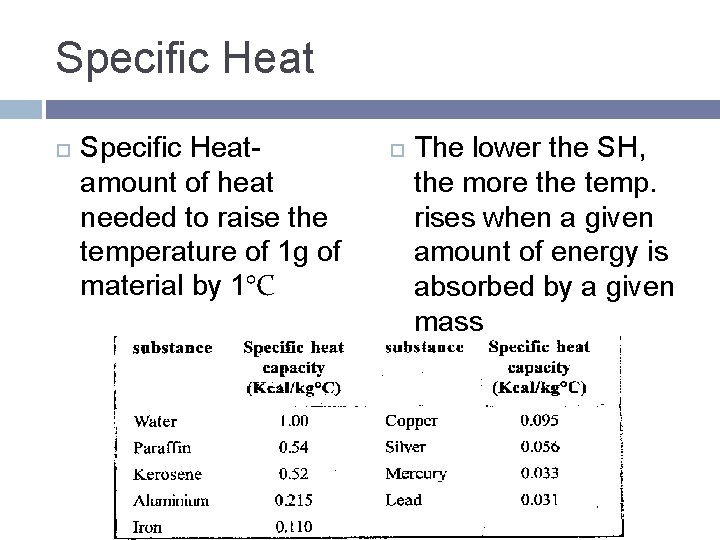
Specific Heat Specific Heatamount of heat needed to raise the temperature of 1 g of material by 1°C The lower the SH, the more the temp. rises when a given amount of energy is absorbed by a given mass

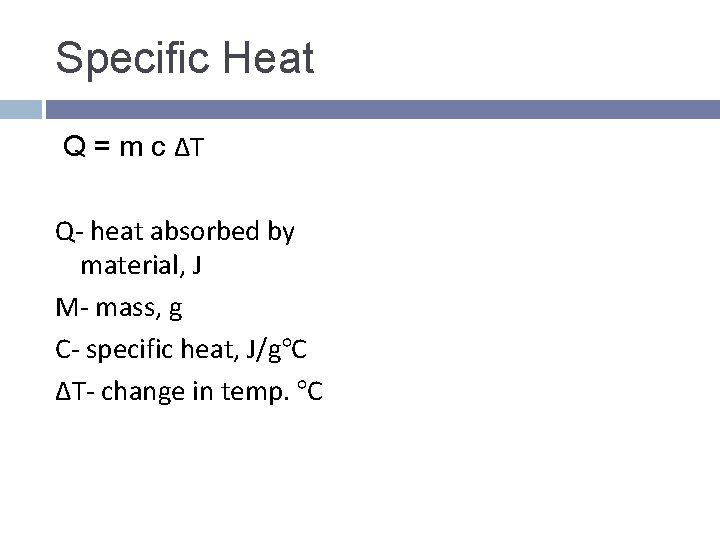
Specific Heat Q = m c ΔT Q- heat absorbed by material, J M- mass, g C- specific heat, J/g°C ΔT- change in temp. °C

Specific Heat Practice An iron skillet has a mass of 500 g. Its specific heat is 0. 449 J/g°C. How much heat must be absorbed to raise the temperature by 95 C? Given: Formula: Solve:

Math Practice pg 477 2. 3. 4. 5.

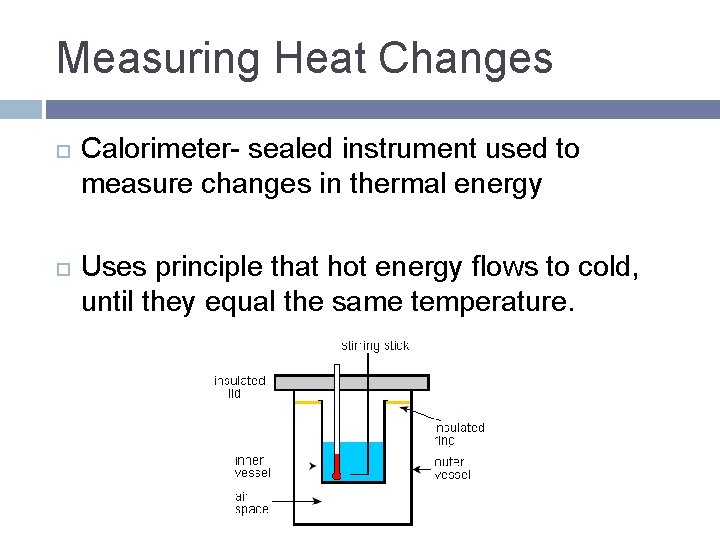
Measuring Heat Changes Calorimeter- sealed instrument used to measure changes in thermal energy Uses principle that hot energy flows to cold, until they equal the same temperature.

HEAT AND THERMODYNAMICS Ch. 16. 2

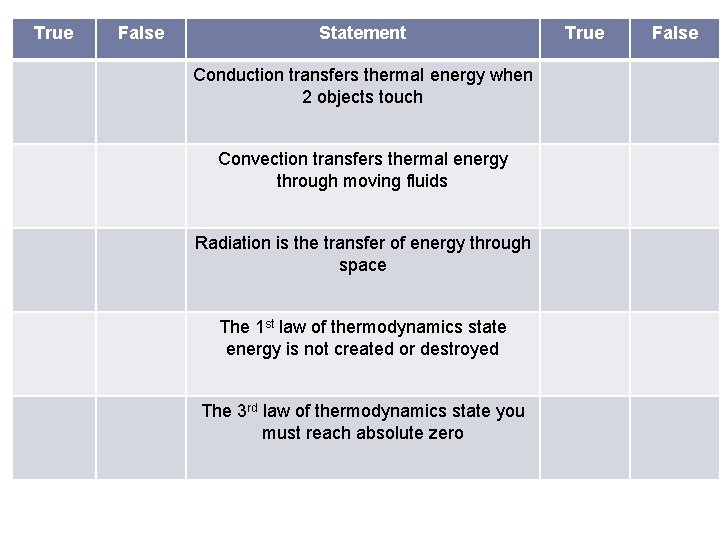
True False Statement Conduction transfers thermal energy when 2 objects touch Convection transfers thermal energy through moving fluids Radiation is the transfer of energy through space The 1 st law of thermodynamics state energy is not created or destroyed The 3 rd law of thermodynamics state you must reach absolute zero True False

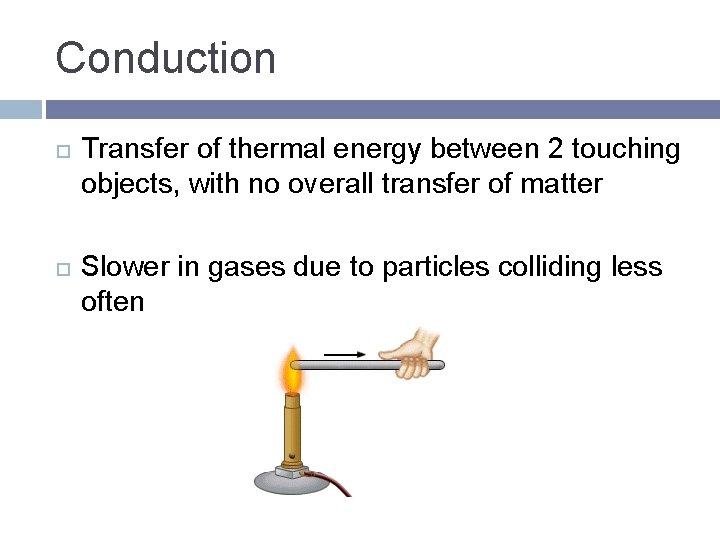
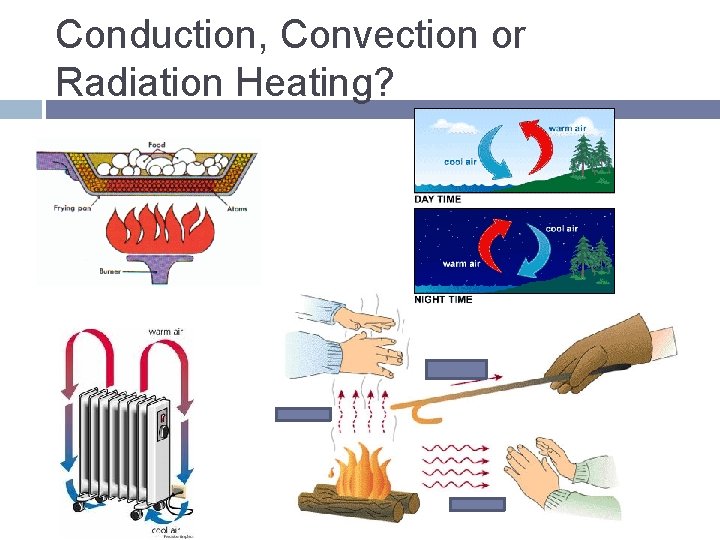
Conduction Transfer of thermal energy between 2 touching objects, with no overall transfer of matter Slower in gases due to particles colliding less often

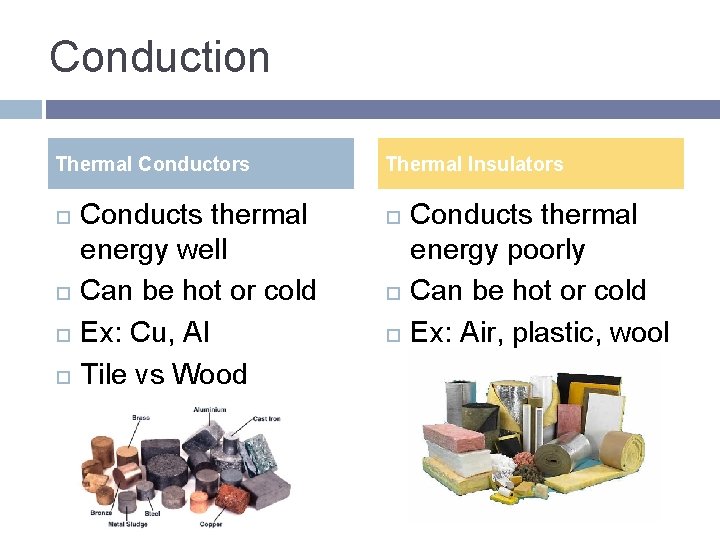
Conduction Thermal Conductors Conducts thermal energy well Can be hot or cold Ex: Cu, Al Tile vs Wood Thermal Insulators Conducts thermal energy poorly Can be hot or cold Ex: Air, plastic, wool

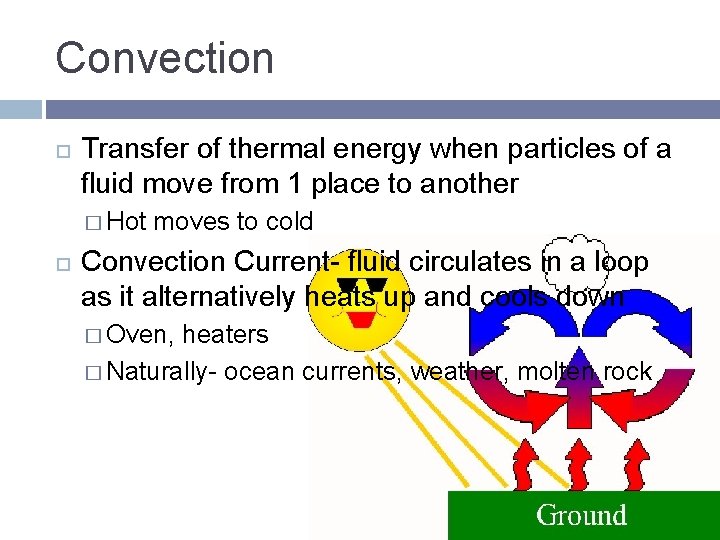
Convection Transfer of thermal energy when particles of a fluid move from 1 place to another � Hot moves to cold Convection Current- fluid circulates in a loop as it alternatively heats up and cools down � Oven, heaters � Naturally- ocean currents, weather, molten rock

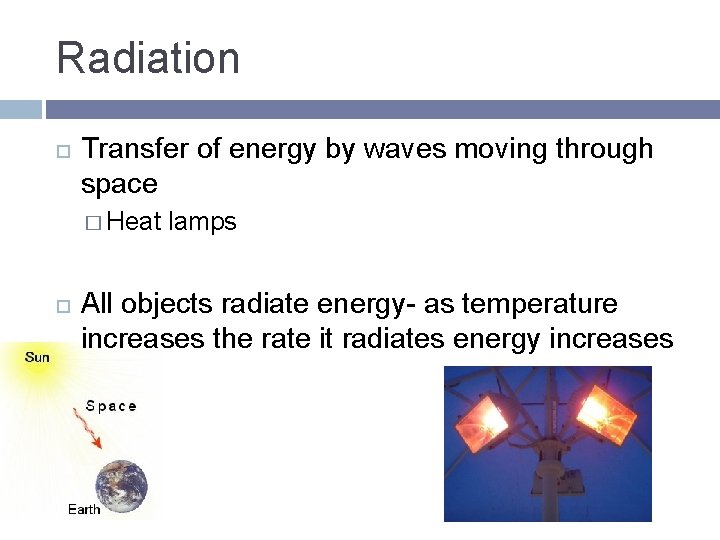
Radiation Transfer of energy by waves moving through space � Heat lamps All objects radiate energy- as temperature increases the rate it radiates energy increases

Conduction, Convection or Radiation Heating?

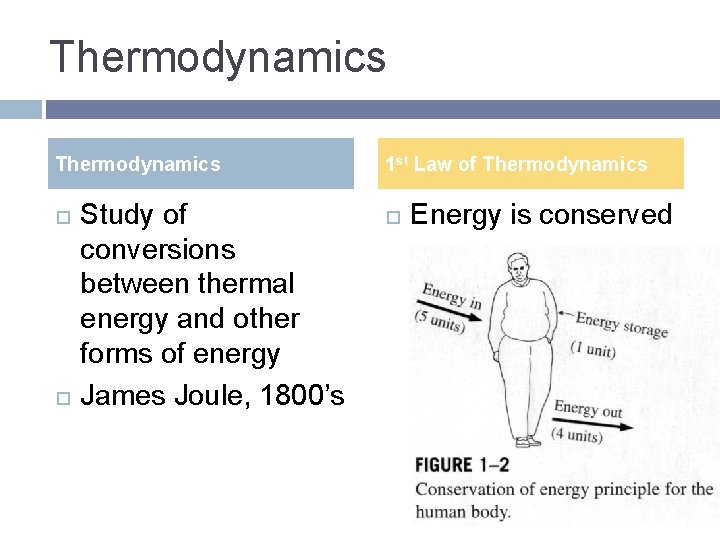
Thermodynamics Study of conversions between thermal energy and other forms of energy James Joule, 1800’s 1 st Law of Thermodynamics Energy is conserved

Thermodynamics 2 nd Law of Thermodynamics Thermal energy can flow from cold to hot objects ONLY if work is done to the system Heat engine- device converts heat into work Waste heat- thermal energy not converted into heat 4 th Law of Thermodynamics Absolute zero cannot be reached

USING HEAT Ch. 16. 3

True False Statement Heat engines can be internal or external, but neither are very efficient Hot water, steam and electric, and forced air heating all use conduction and radiation to heat Heat pumps reverse the normal flow of thermal energy, which is hot to cold Refrigerators and air conditioners are cooling systems, that don’t require heat pumps Refrigerants vaporize and condense over and over again True False

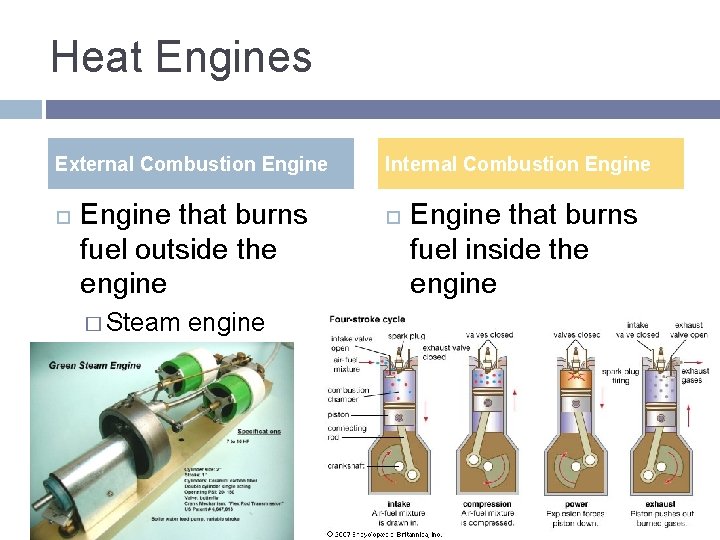
Heat Engines External Combustion Engine that burns fuel outside the engine � Steam engine Internal Combustion Engine that burns fuel inside the engine � Cars

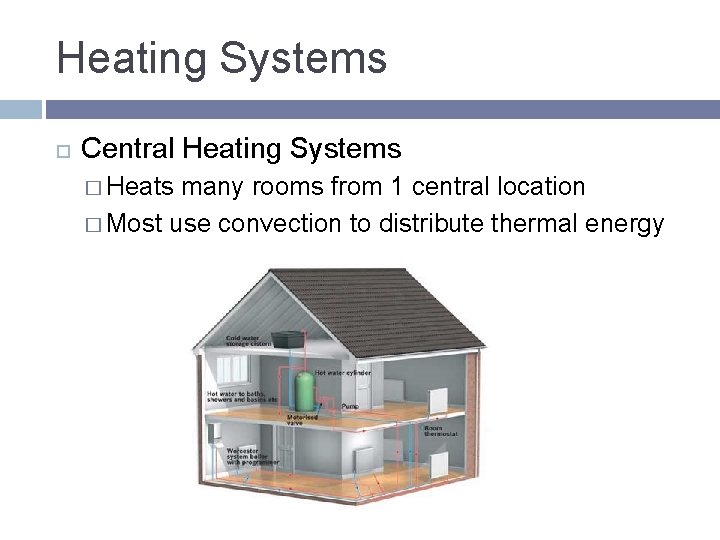
Heating Systems Central Heating Systems � Heats many rooms from 1 central location � Most use convection to distribute thermal energy

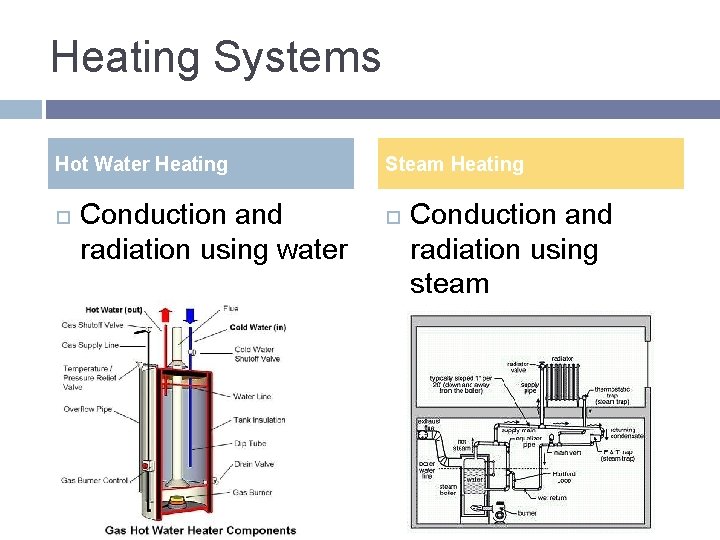
Heating Systems Hot Water Heating Conduction and radiation using water Steam Heating Conduction and radiation using steam

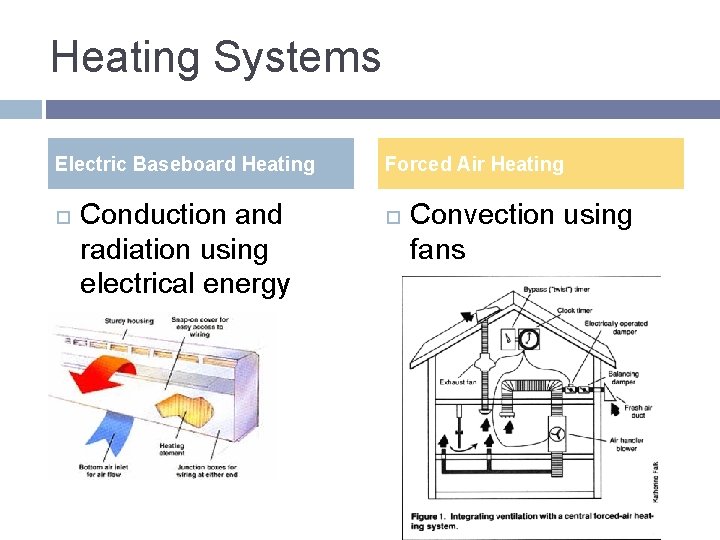
Heating Systems Electric Baseboard Heating Conduction and radiation using electrical energy Forced Air Heating Convection using fans

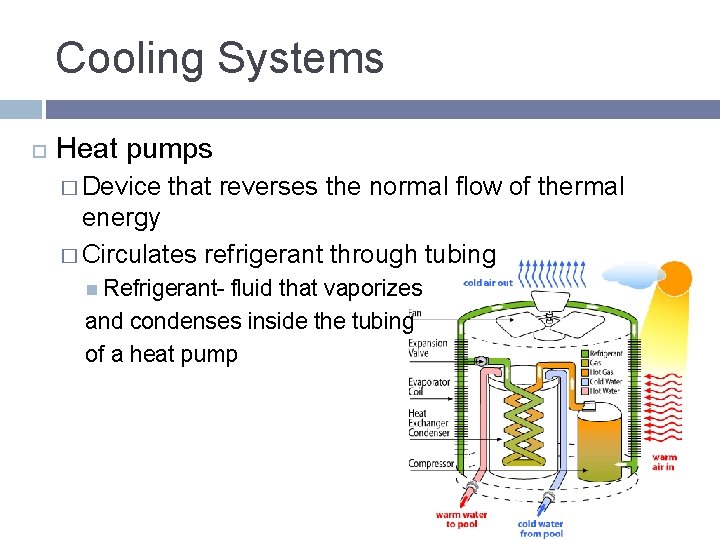
Cooling Systems Heat pumps � Device that reverses the normal flow of thermal energy � Circulates refrigerant through tubing Refrigerant- fluid that vaporizes and condenses inside the tubing of a heat pump

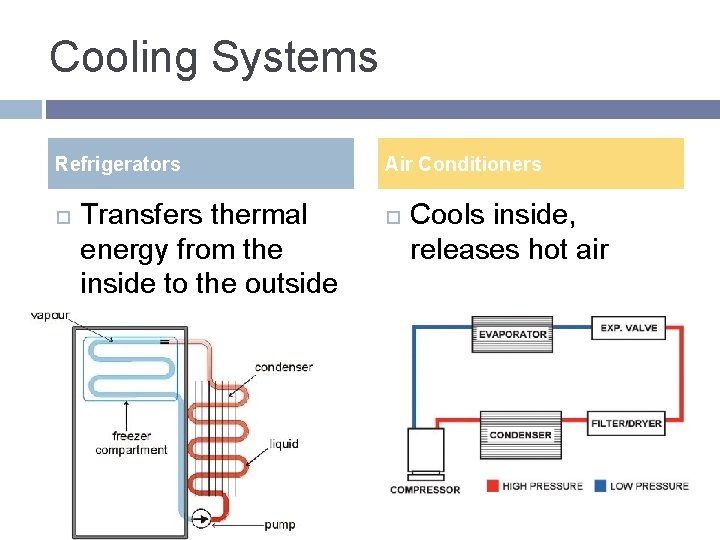
Cooling Systems Refrigerators Transfers thermal energy from the inside to the outside Air Conditioners Cools inside, releases hot air
 Section 3 using heat worksheet answers
Section 3 using heat worksheet answers Section 16.1 thermal energy and matter
Section 16.1 thermal energy and matter Section 1 matter and thermal energy
Section 1 matter and thermal energy Lesson 1 thermal energy and the behavior of matter
Lesson 1 thermal energy and the behavior of matter Thermal energy in states of matter
Thermal energy in states of matter Heat thermal energy and temperature
Heat thermal energy and temperature Thermal energy in states of matter
Thermal energy in states of matter Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter How are thermal energy and temperature different
How are thermal energy and temperature different Kinetic energy to thermal energy
Kinetic energy to thermal energy Thermal transfer vs direct thermal printing
Thermal transfer vs direct thermal printing Chapter 2 jesus christ true god and true man
Chapter 2 jesus christ true god and true man Eating food physical or chemical change
Eating food physical or chemical change Gray matter
Gray matter What makes up the diencephalon
What makes up the diencephalon Gray matter and white matter
Gray matter and white matter Telencephalon
Telencephalon Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Difference between heat and thermal energy
Difference between heat and thermal energy Specific heat capacity of lead j/kg c
Specific heat capacity of lead j/kg c Difference between heat and thermal energy
Difference between heat and thermal energy Flannel shirt conductor or insulator
Flannel shirt conductor or insulator Heat thermal energy and temperature
Heat thermal energy and temperature Heat thermal energy and temperature
Heat thermal energy and temperature Mass and thermal energy
Mass and thermal energy Chapter 16 thermal energy and heat
Chapter 16 thermal energy and heat Amer rasheed
Amer rasheed Section 1 composition of matter
Section 1 composition of matter