Thermal Energy n Thermal energy kinetic energy of








- Slides: 22

Thermal Energy

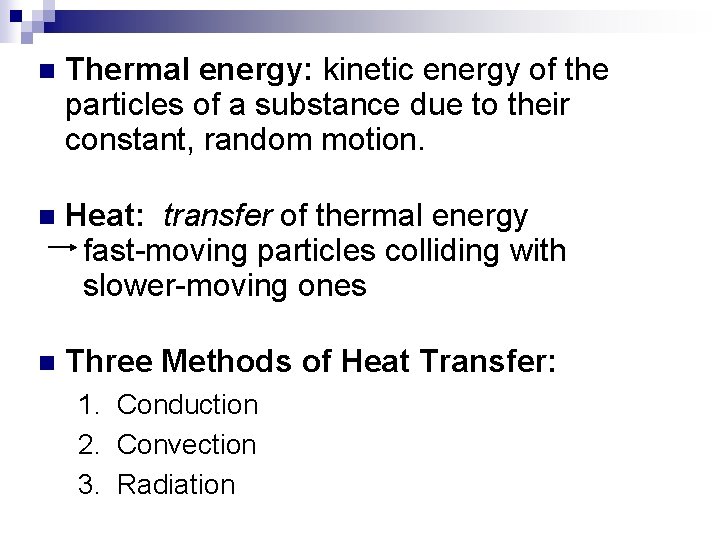
n Thermal energy: kinetic energy of the particles of a substance due to their constant, random motion. n Heat: n Three Methods of Heat Transfer: 1 2. 3.

n Thermal energy: kinetic energy of the particles of a substance due to their constant, random motion. n Heat: transfer of thermal energy fast-moving particles colliding with slower-moving ones n Three Methods of Heat Transfer: 1. Conduction 2. Convection 3. Radiation

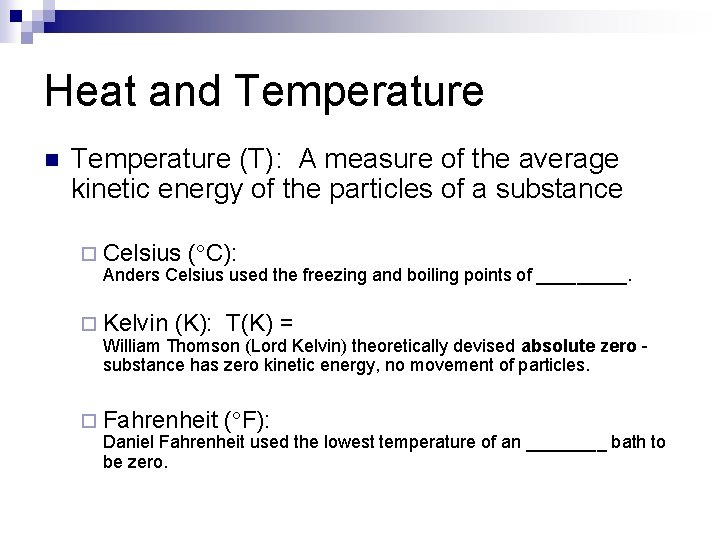
Heat and Temperature n Temperature (T): A measure of the average kinetic energy of the particles of a substance ¨ Celsius ( C): Anders Celsius used the freezing and boiling points of _____. ¨ Kelvin (K): T(K) = William Thomson (Lord Kelvin) theoretically devised absolute zero substance has zero kinetic energy, no movement of particles. ¨ Fahrenheit ( F): Daniel Fahrenheit used the lowest temperature of an ____ bath to be zero.

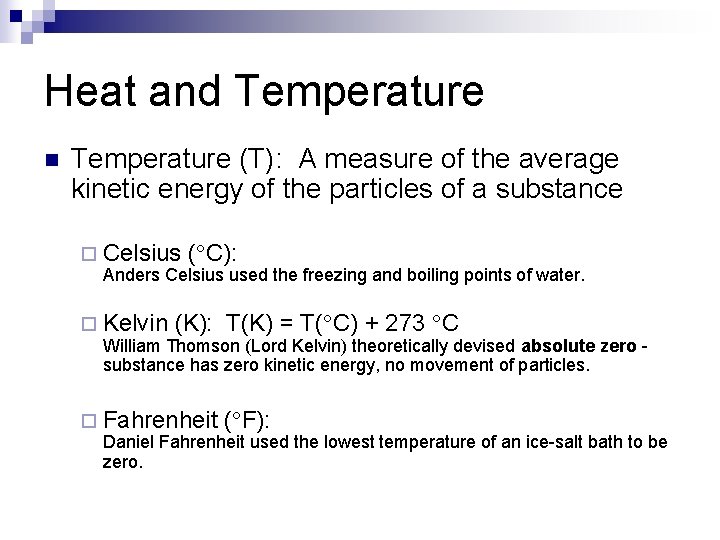
Heat and Temperature n Temperature (T): A measure of the average kinetic energy of the particles of a substance ¨ Celsius ( C): Anders Celsius used the freezing and boiling points of water. ¨ Kelvin (K): T(K) = T( C) + 273 C William Thomson (Lord Kelvin) theoretically devised absolute zero substance has zero kinetic energy, no movement of particles. ¨ Fahrenheit ( F): Daniel Fahrenheit used the lowest temperature of an ice-salt bath to be zero.

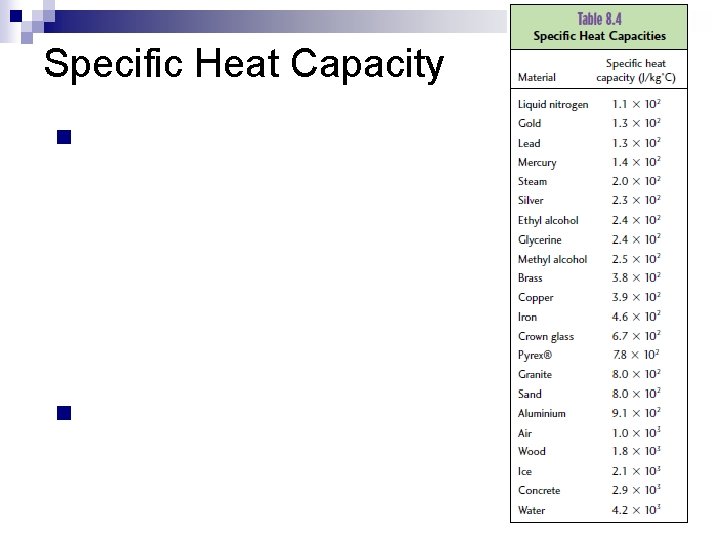
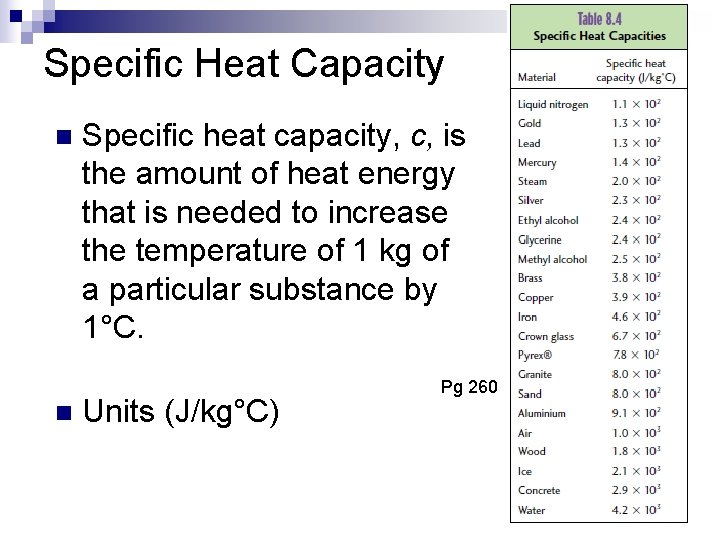
Specific Heat Capacity n n

Specific Heat Capacity n n Specific heat capacity, c, is the amount of heat energy that is needed to increase the temperature of 1 kg of a particular substance by 1°C. Units (J/kg°C) Pg 260

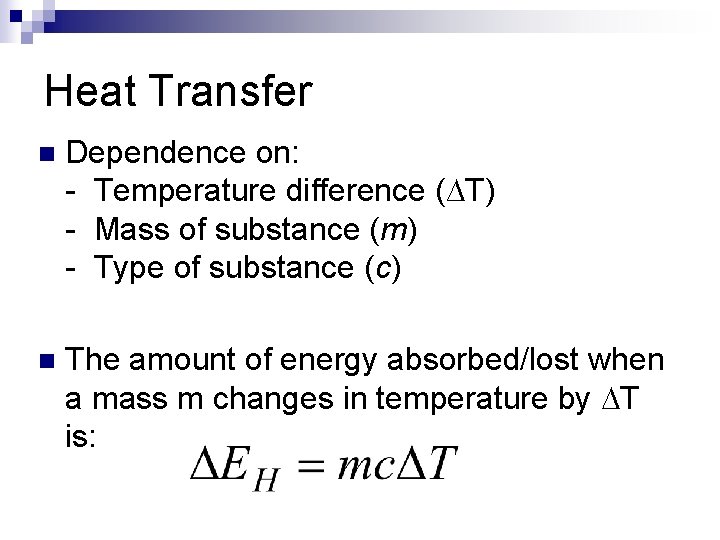
Heat Transfer n Dependence on: - Temperature difference (∆T) - Mass of substance (m) - Type of substance (c) n The amount of energy absorbed/lost when a mass m changes in temperature by T is:

Heat Transfer n Dependence on: - Temperature difference (∆T) - Mass of substance (m) - Type of substance (c) n The amount of energy absorbed/lost when a mass m changes in temperature by T is:

n A 0. 50 kg block of iron at 80. 0°C is cooled by removing 2. 28 x 104 J of heat energy. What will the final temperature of the metal be?

Thermal Energy

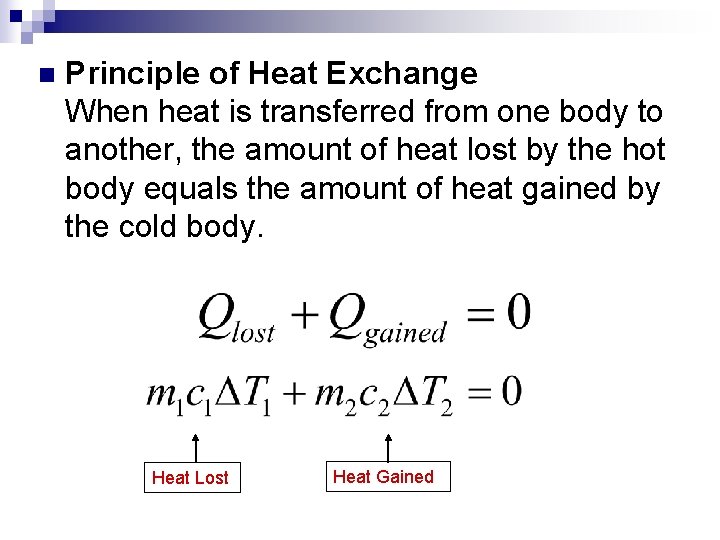
n Principle of Heat Exchange When heat is transferred from one body to another, the amount of heat lost by the hot body equals the amount of heat gained by the cold body.

n Principle of Heat Exchange When heat is transferred from one body to another, the amount of heat lost by the hot body equals the amount of heat gained by the cold body. Heat Lost Heat Gained

n Red & Blue: 0. 80 kg of red hot water at 90°C is allowed to mix with 0. 70 kg of cold blue water at 5°C. a) What will happen when the divider is removed? b) What is the final temperature of the mixture?

n A 200 -g piece of iron was heated and was submerged in 150 g of water at 20°C to be cooled quickly. The final temperature of the iron and the water is _____°C. Determine the initial temperature of the iron. (Assume two significant digits. )

Calorimetry the careful and precise measurement of heat transfer n 1 cal = 4. 1858 J n

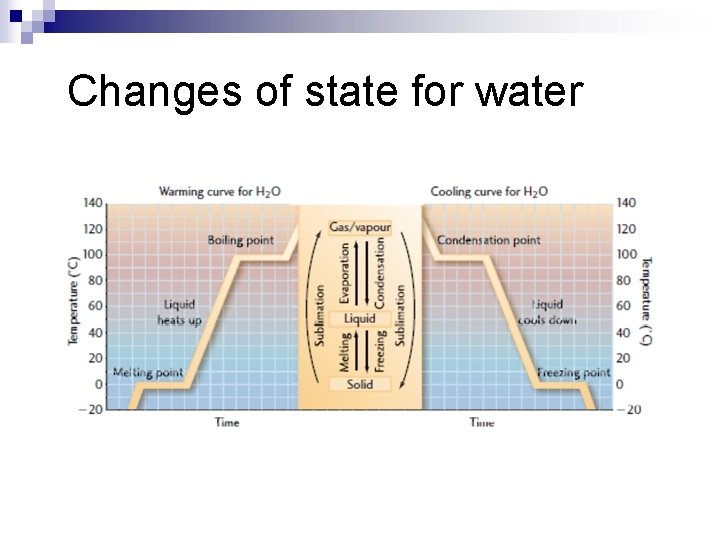
Changes of State and Latent Heat

Changes of state for water

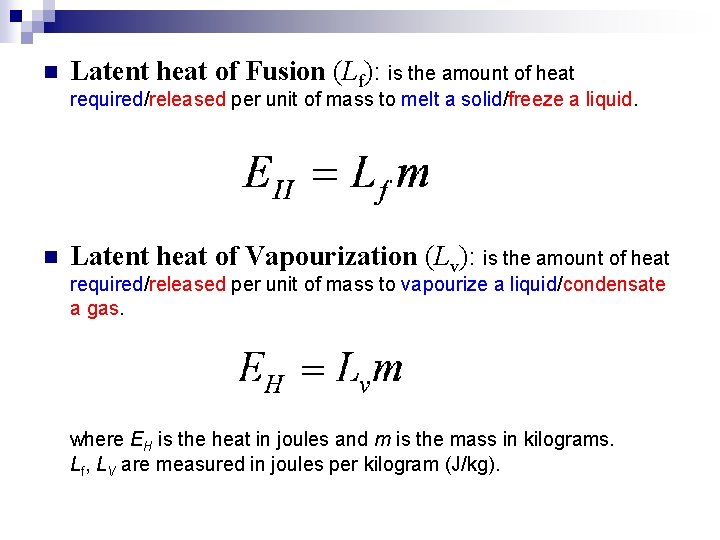
n Latent heat of Fusion (Lf): is the amount of heat required/released per unit of mass to melt a solid/freeze a liquid. n Latent heat of Vapourization (Lv): is the amount of heat required/released per unit of mass to vapourize a liquid/condensate a gas. where EH is the heat in joules and m is the mass in kilograms. Lf, LV are measured in joules per kilogram (J/kg).

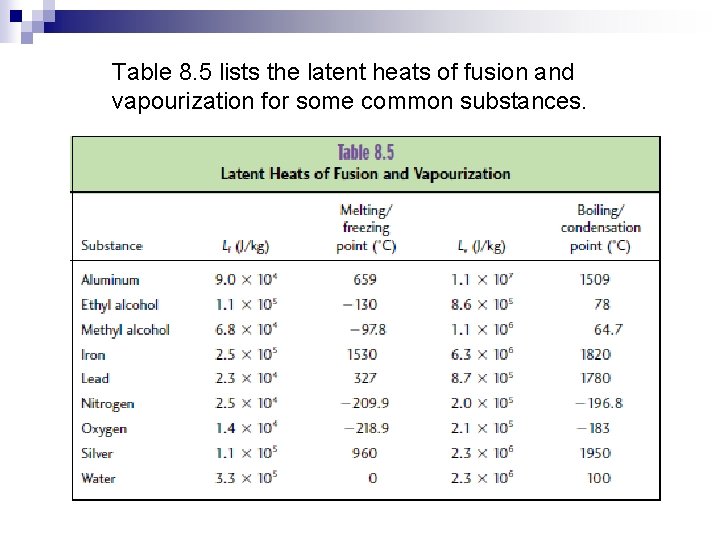
Table 8. 5 lists the latent heats of fusion and vapourization for some common substances.

n Ex. One danger of leaving a tea kettle unattended on the stove is having it boil dry. How much heat is required to change 0. 20 kg of water (about the amount for a good cup of tea) from room temperature of 20ºC to vapour? Calculate the cost if electricity in Ontario cost around $0. 12 k. W. h. [5. 3 x 105 J, $0. 02]

n What would be the total amount of energy released in cooling 3. 00 kg of nitrogen gas at room temperature, 20. 0ºC to liquid nitrogen at -201ºC? ¨ c. N-gas = 1. 04 x 103 J/kgo. C; c. N-liquid = 1. 10 x 103 J/kgo. C