Ch 5 Thermal Energy and Heat Thermal Energy














- Slides: 33

Ch 5 Thermal Energy and Heat

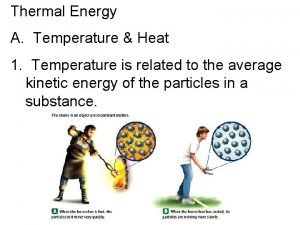
Thermal Energy Temperature & Heat Temperature is a measure of the average kinetic energy of the individual particles in a substance.

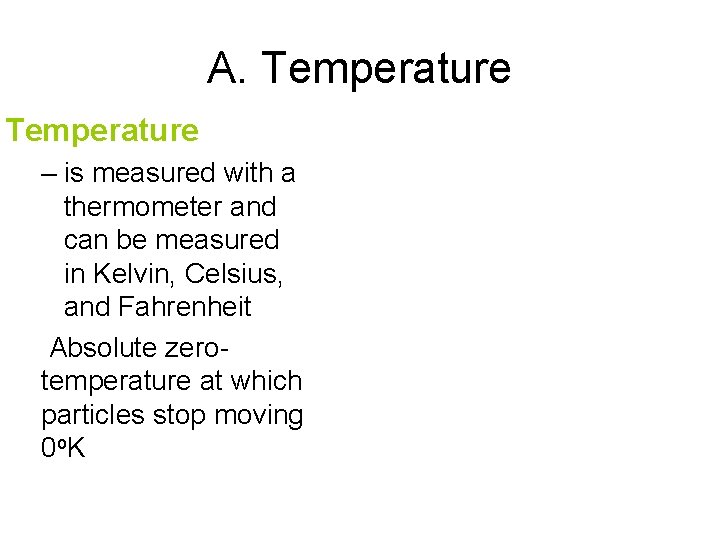
A. Temperature – is measured with a thermometer and can be measured in Kelvin, Celsius, and Fahrenheit Absolute zerotemperature at which particles stop moving 0 o. K

2. Temperature Conversions F= 1. 8 C + 32 C= (F-32)/1. 8 What is 78 o. F = _______o. C What is 18 o. F = _______o. C

3. SI unit for temp. is the Kelvin a. K = C + 273 (10 C = 283 K) b. C = K – 273 (10 K = -263 C) B. Thermal Energy – the total of all the kinetic and potential energy of all the particles in a substance.

1. Thermal energy relationships a. Depends on temperature, mass, and type of substance b. As temperature increases, so does thermal energy (because the kinetic energy of the particles increased). c. Even if the temperature doesn’t change, thermal energy of a more massive substance is higher (because it is a total measure of energy).

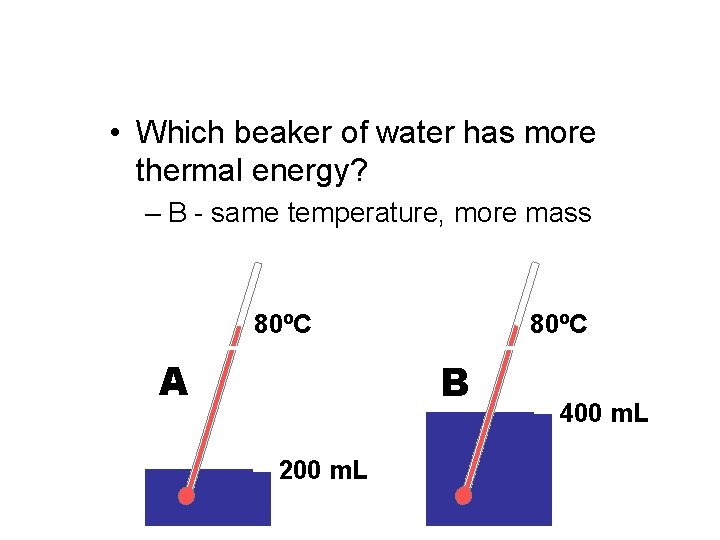
• Which beaker of water has more thermal energy? – B - same temperature, more mass 80ºC A 80ºC B 200 m. L 400 m. L

2. Heat Cup gets cooler while hand gets warmer a. The flow of thermal energy from one object to another. b. Heat always flows from warmer to cooler objects. Ice gets warmer while hand gets cooler

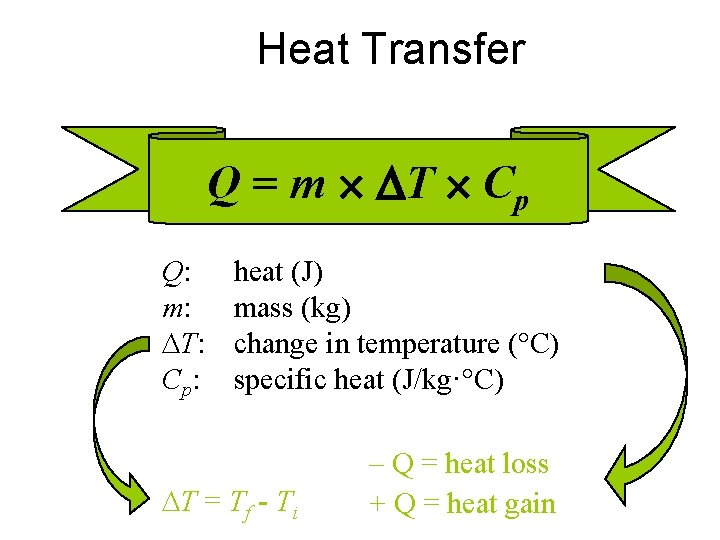
C. Heat Transfer 1. Specific Heat (Cp) – amount of energy required to raise the temp. of 1 kg of material by 1 degree Kelvin – units: J/(kg·K) or J/(kg·°C) or J/(g·°C) – MUST PAY ATTENTION TO UNITS

Heat Transfer • Which sample will take longer to heat to 100°C? 50 g Al 50 g Cu • Al - It has a higher specific heat. • Al will also take longer to cool down.

Heat Transfer Q = m T Cp Q: m: T: Cp: heat (J) mass (kg) change in temperature (°C) specific heat (J/kg·°C) T = Tf - Ti – Q = heat loss + Q = heat gain

Specific Heat 2. Some things heat up or cool down faster than others. Land heats up and cools down faster than water

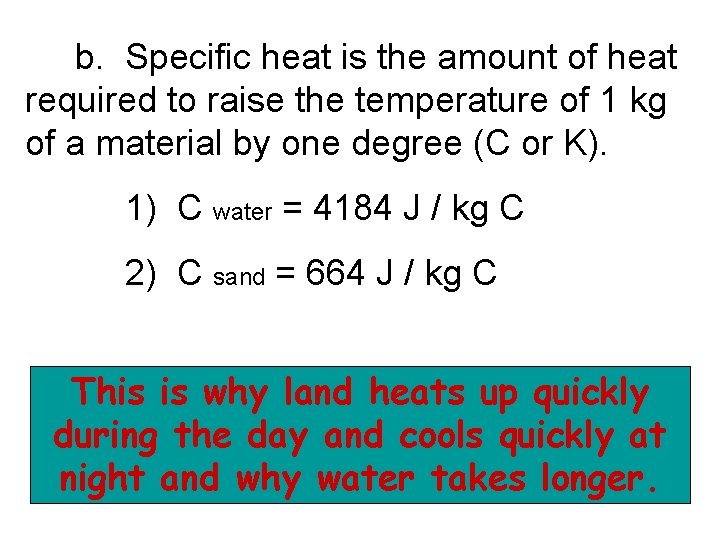
b. Specific heat is the amount of heat required to raise the temperature of 1 kg of a material by one degree (C or K). 1) C water = 4184 J / kg C 2) C sand = 664 J / kg C This is why land heats up quickly during the day and cools quickly at night and why water takes longer.

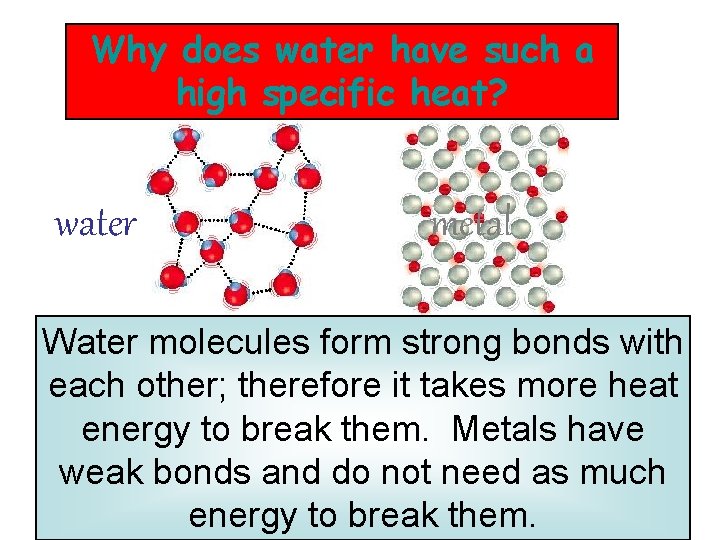
Why does water have such a high specific heat? water metal Water molecules form strong bonds with each other; therefore it takes more heat energy to break them. Metals have weak bonds and do not need as much energy to break them.

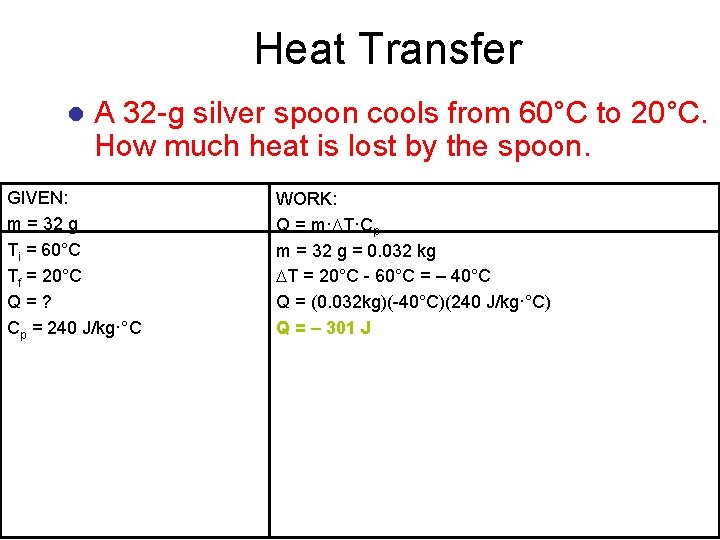
Heat Transfer l A 32 -g silver spoon cools from 60°C to 20°C. How much heat is lost by the spoon. ? GIVEN: m = 32 g Ti = 60°C Tf = 20°C Q=? Cp = 240 J/kg·°C WORK: Q = m· T·Cp m = 32 g = 0. 032 kg T = 20°C - 60°C = – 40°C Q = (0. 032 kg)(-40°C)(240 J/kg·°C) Q = – 301 J

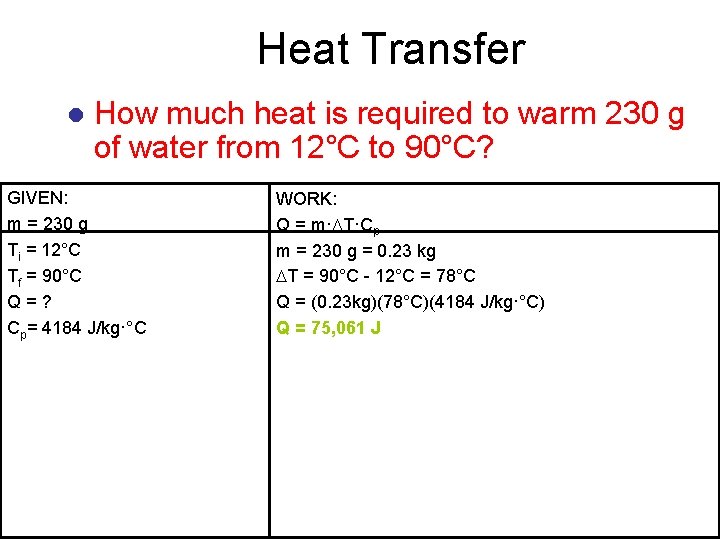
Heat Transfer l How much heat is required to warm 230 g of water from 12°C to 90°C? GIVEN: m = 230 g Ti = 12°C Tf = 90°C Q=? Cp= 4184 J/kg·°C WORK: Q = m· T·Cp m = 230 g = 0. 23 kg T = 90°C - 12°C = 78°C Q = (0. 23 kg)(78°C)(4184 J/kg·°C) Q = 75, 061 J

6. 2 The Transfer of Heat

A. How is heat transferred? l What type of HEAT TRANSFER is occurring in the pictures? Conduction, convection or radiation? CONDUCTION – The transfer of thermal energy with no transfer of matter. Occurs because particles that make up matter are in constant motion

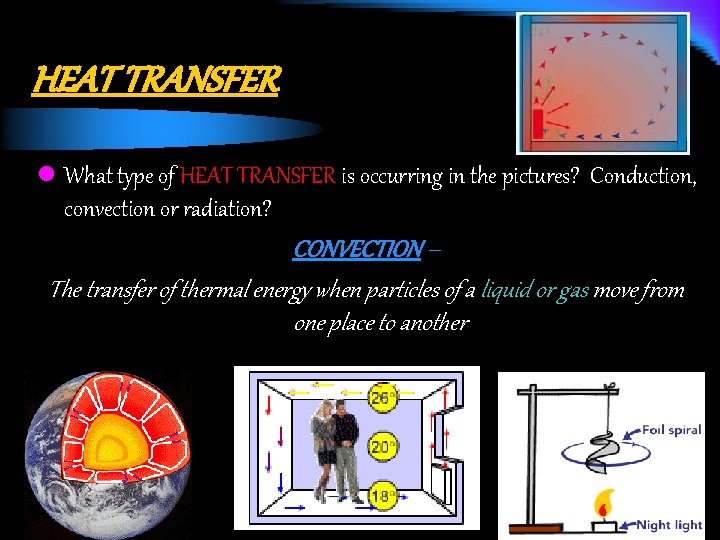
HEAT TRANSFER l What type of HEAT TRANSFER is occurring in the pictures? Conduction, convection or radiation? CONVECTION – The transfer of thermal energy when particles of a liquid or gas move from one place to another

HEAT TRANSFER CONVECTION – in the earth and sun The circular flow of hot and cold creates convection currents

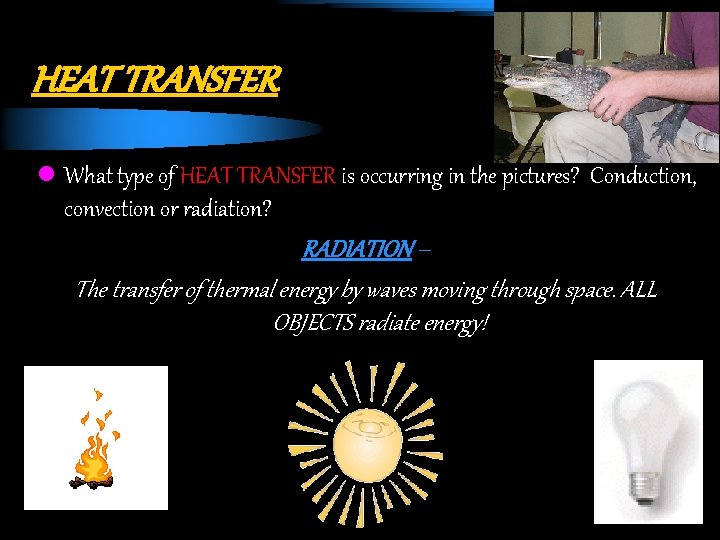
HEAT TRANSFER l What type of HEAT TRANSFER is occurring in the pictures? Conduction, convection or radiation? RADIATION – The transfer of thermal energy by waves moving through space. ALL OBJECTS radiate energy!

B. Conductors and Insulators Materials are either conductors or insulators. A conductor transfers thermal energy Ex: metals-silver and steel, tile floors takes heat away from your An insulator does not transfer thermal energy well. Ex: wood, wool, straw, paper

THERMAL ENERGY & MATTER: Exit Slip Define Convection, Conduction and Radiation 2. Give an example of each. 3. Write a sentence describing how each is important to our everyday lives. 4. How do we use heat in our everyday lives? 1.

Section 3 – Using Heat l Heating Systems u Campfire u Furnace u Radiator u Electric heating u Solar heating

Thermodynamics l Study of the relationships between thermal energy, heat and work u Heat and work increase thermal energy u Heat – warming hands by a fire u Work – warming hands by rubbing them together

1 st Law of Thermodynamics l If the mechanical energy of a system is constant, the increase in thermal energy of that system equals the sum of thermal energy transfers into that systems and the work done on that system l https: //www. khanacademy. org/science/biology/energy -and-enzymes/the-laws-of-thermodynamics/v/first-law -of-thermodynamics-introduction

2 nd Law of Thermodynamics l Energy spontaneously spreads from regions of higher concentration to regions of lower concentration l https: //www. khanacademy. org/science/biology/energy -and-enzymes/the-laws-of-thermodynamics/v/thesecond-law-of-thermodynamics

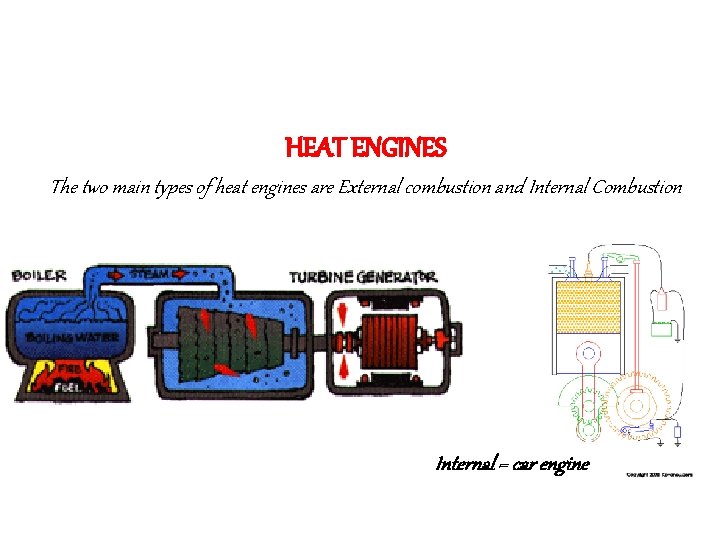
Section 4 Uses of Heat A. Heat engine- A device that transforms thermal energy to mechanical energy. l Classified according to whether combustion takes place outside or inside the engine. l Usually through combustion.

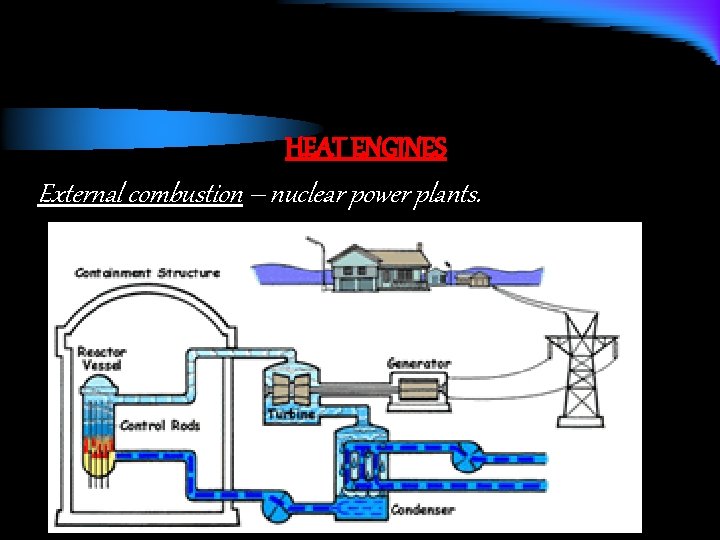
HEAT ENGINES The two main types of heat engines are External combustion and Internal Combustion External = power plants Internal = car engine

External combustion – burn fuel outside the engine in a boiler Examples: power plants, steam engine Water is heated by a fuel and the pressurized steam spins a turbine. http: //www. eas. asu. edu/~holbert/eee 463/coal. html

HEAT ENGINES External combustion – nuclear power plants.

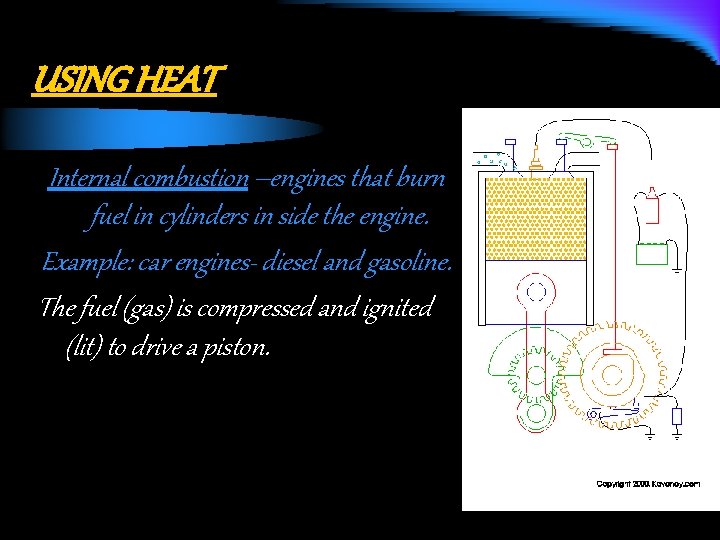
USING HEAT Internal combustion –engines that burn fuel in cylinders in side the engine. Example: car engines- diesel and gasoline. The fuel (gas) is compressed and ignited (lit) to drive a piston.

B. Cooling Systems • Refrigerator-transfers thermal energy from inside the refrigerator to the room outside. • The refrigerant absorbs and releases heat. • http: //www. youtube. com/watch? v=BFt. Q 7 Xv. Axc • Air conditioner-absorb heat from the air inside a room or car and transfers it outdoors.
 Section 3 using thermal energy answers
Section 3 using thermal energy answers Thermal energy vs heat energy
Thermal energy vs heat energy Difference between heat and thermal energy
Difference between heat and thermal energy Specific heat capacity of lead j/kg c
Specific heat capacity of lead j/kg c Difference between heat and thermal energy
Difference between heat and thermal energy What is heat energy?
What is heat energy? What is the difference between thermal energy and heat?
What is the difference between thermal energy and heat? Heat thermal energy and temperature
Heat thermal energy and temperature Heat thermal energy and temperature
Heat thermal energy and temperature Chapter 16 thermal energy and heat
Chapter 16 thermal energy and heat Energy and temperature
Energy and temperature Heat vs thermal energy
Heat vs thermal energy Thermal energy vs heat
Thermal energy vs heat Heat vs thermal energy vs temperature
Heat vs thermal energy vs temperature Is temperature a measure of thermal energy
Is temperature a measure of thermal energy Kinetic energy to thermal energy
Kinetic energy to thermal energy Thermal transfer vs direct thermal printing
Thermal transfer vs direct thermal printing Latent heat dimension
Latent heat dimension Potential energy formula
Potential energy formula Properties of heat
Properties of heat Dry heat cooking methods examples
Dry heat cooking methods examples Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Chapter 5 thermal energy
Chapter 5 thermal energy Which reverses the normal flow of thermal energy
Which reverses the normal flow of thermal energy Matter and thermal energy section 1
Matter and thermal energy section 1 The science duo physical and chemical changes
The science duo physical and chemical changes Solar energy is radiant light and heat
Solar energy is radiant light and heat How does sound travel
How does sound travel Heat and temperature relationship
Heat and temperature relationship Heat and internal energy
Heat and internal energy How is thermal energy transferred?
How is thermal energy transferred? Thermal energy depends on
Thermal energy depends on Whats thermal energy
Whats thermal energy