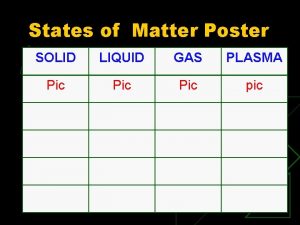
States of Matter SOLID LIQUID GAS Thermal Energy














- Slides: 29

States of Matter SOLID LIQUID GAS

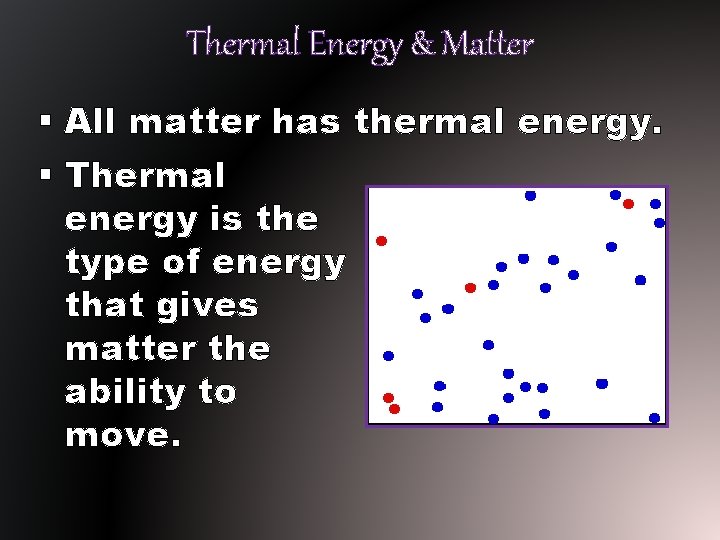
Thermal Energy & Matter § All matter has thermal energy. § Thermal energy is the type of energy that gives matter the ability to move.

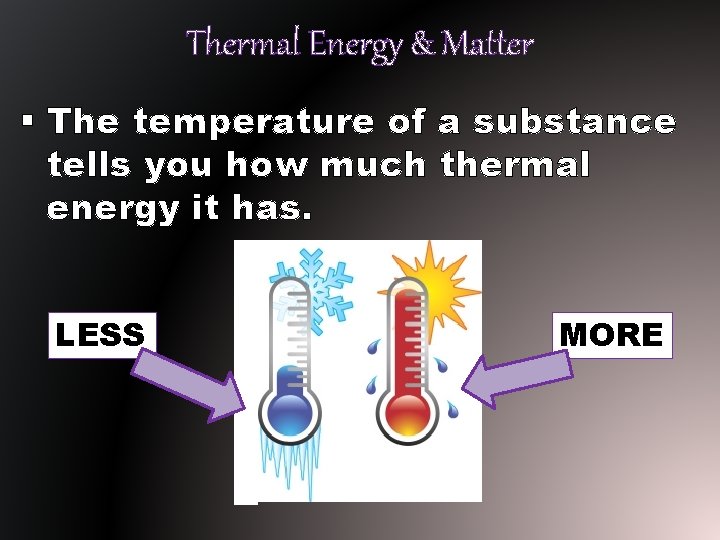
Thermal Energy & Matter § The temperature of a substance tells you how much thermal energy it has. LESS MORE

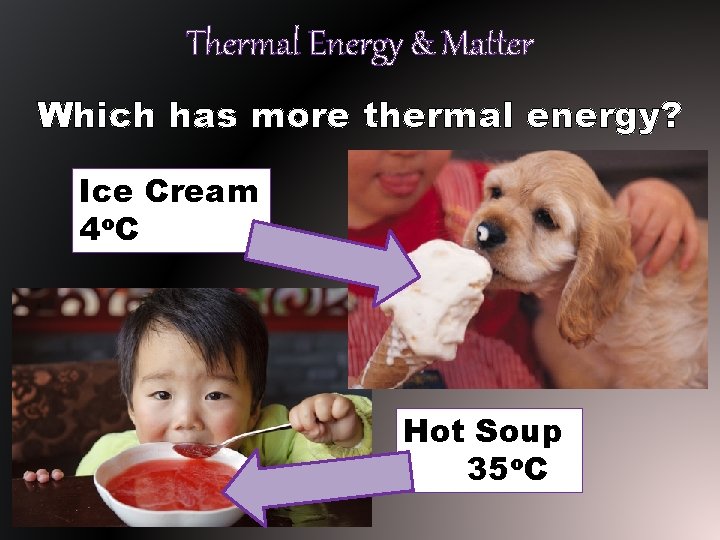
Thermal Energy & Matter Which has more thermal energy? Ice Cream 4 o. C Hot Soup 35 o. C

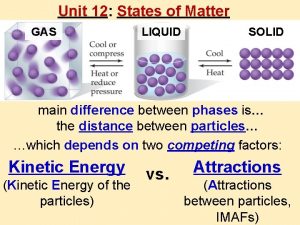
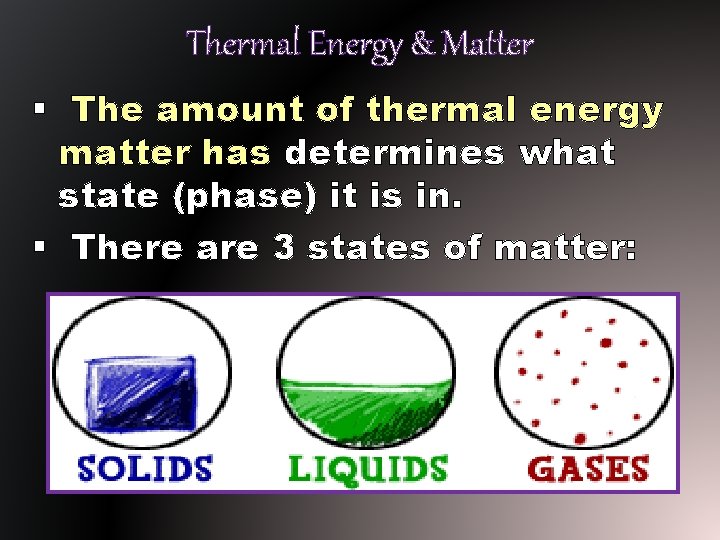
Thermal Energy & Matter § The amount of thermal energy matter has determines what state (phase) it is in. § There are 3 states of matter:

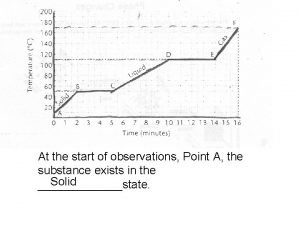
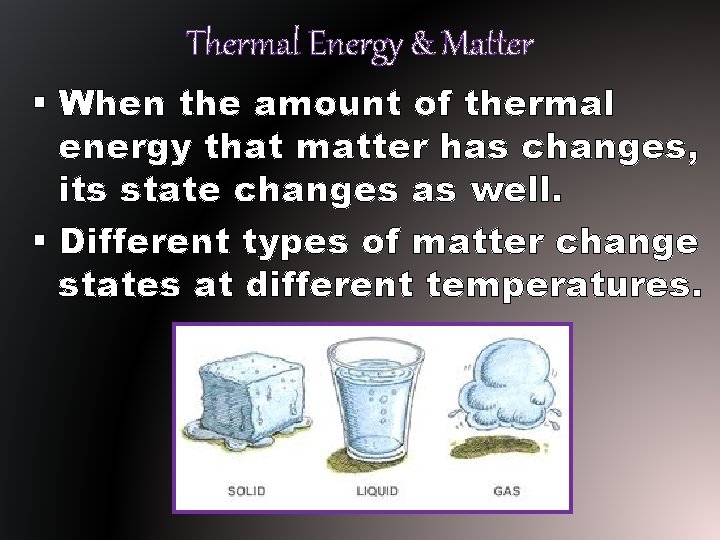
Thermal Energy & Matter § When the amount of thermal energy that matter has changes, its state changes as well. § Different types of matter change states at different temperatures.

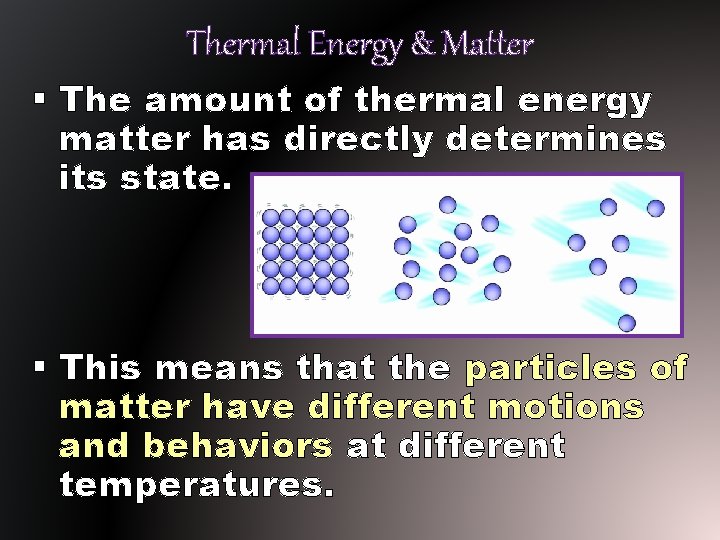
Thermal Energy & Matter § The amount of thermal energy matter has directly determines its state. § This means that the particles of matter have different motions and behaviors at different temperatures.

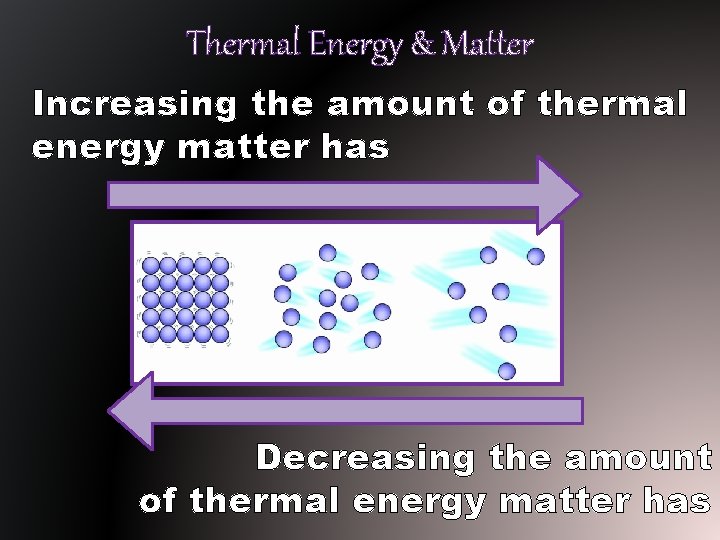
Thermal Energy & Matter Increasing the amount of thermal energy matter has Decreasing the amount of thermal energy matter has

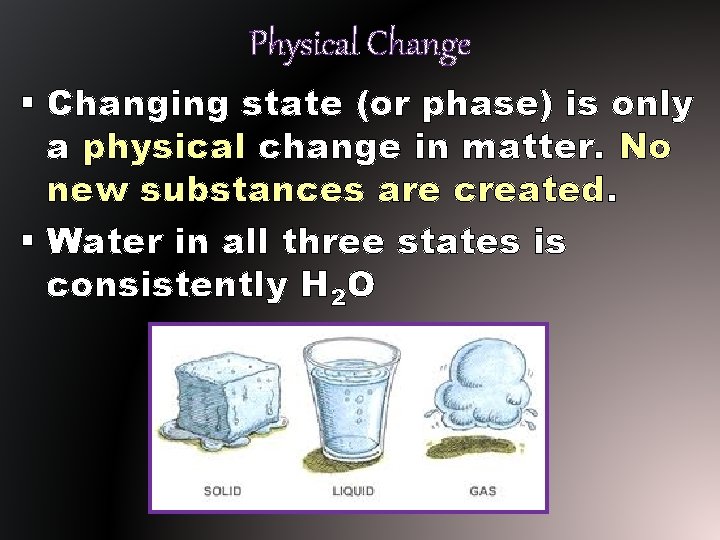
Physical Change § When matter gains or looses thermal energy, it changes its state. § Even though the matter may be in a different state because of thermal energy amounts, it never stops being the same type of matter - only some extensive properties change.

Physical Change § Changing state (or phase) is only a physical change in matter. No new substances are created. § Water in all three states is consistently H 2 O

Matter in Motion § Every atom that makes every type of matter always has some amount of thermal energy – therefore every atom is always in motion. § The amount of energy changes the motion. § The change in motion of matter changes its physical properties.

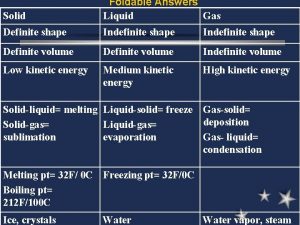
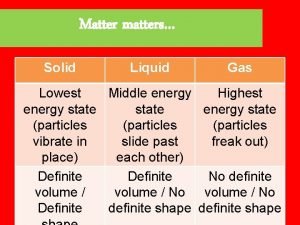
SOLIDS: Thermal Energy § Matter in its solid state has the lowest amount of thermal energy (for that type of matter). § Because solids have less thermal energy than liquids or gasses, the atoms in their solid state move very little.

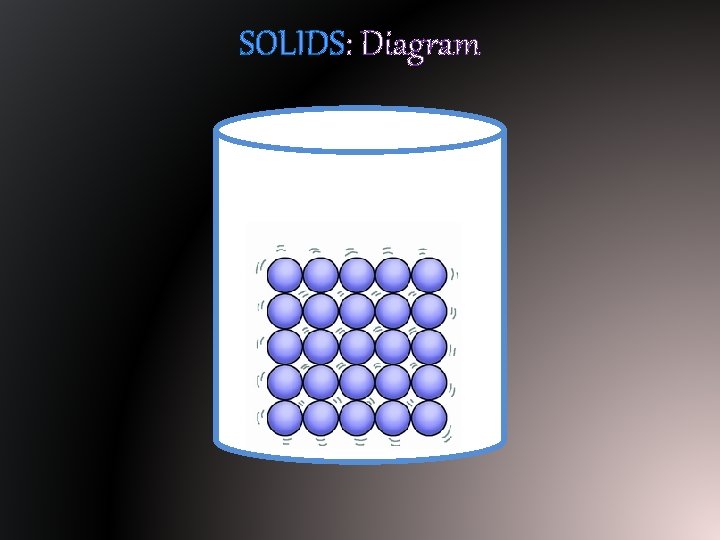
SOLIDS: Particle Motion § Particles (atoms or molecules) of matter in their solid state vibrate in place. § They are packed together and are arranged in a type of pattern.

SOLIDS: Properties § Because matter in its solid state has very little motion, it has specific properties. § Solids have a definite shape. They keep their shape no matter where they are. § Solids have a definite volume – it can be measured (displacement or = x x ) vlwh

SOLIDS: Diagram

LIQUIDS: Thermal Energy § Matter in its liquid state has a medium amount of thermal energy than when it is a solid. § Because liquids have more thermal energy than solids, they move differently than solids.

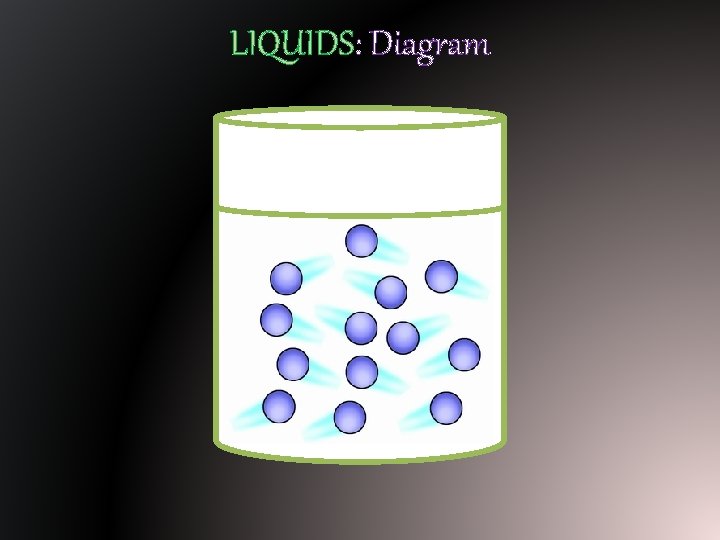
LIQUIDS: Particle Motion § Particles of matter in their liquid state slide past each other. § They are looser and not arranged in any organized way.

LIQUIDS: Properties § Because matter in its liquid has more energy than solids, it has specific properties. § Liquids have no definite shape; they take the shape of their container. § Liquids have a definite volume – it can be measured (graduated cylinder)

LIQUIDS: Diagram

GASSES: Thermal Energy § Matter in its gas state has the most thermal energy than when it is a solid or a liquid. § Because gasses have more thermal energy than others, they move differently than others.

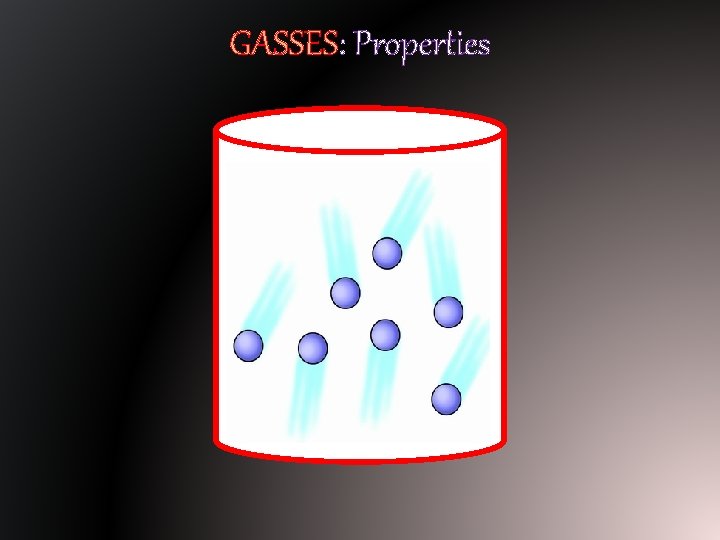
GASSES: Particle Motion § Particles of matter in their gas state bounce everywhere there is open space. § They are even looser than liquids and not arranged in any organized way.

GASSES: Properties § Because matter in its gas state has more energy than solids and liquids, it has specific properties. § Gasses have no definite shape; they take the shape of their container. § Gasses have no definite volume – it can not be directly measured.

GASSES: Properties

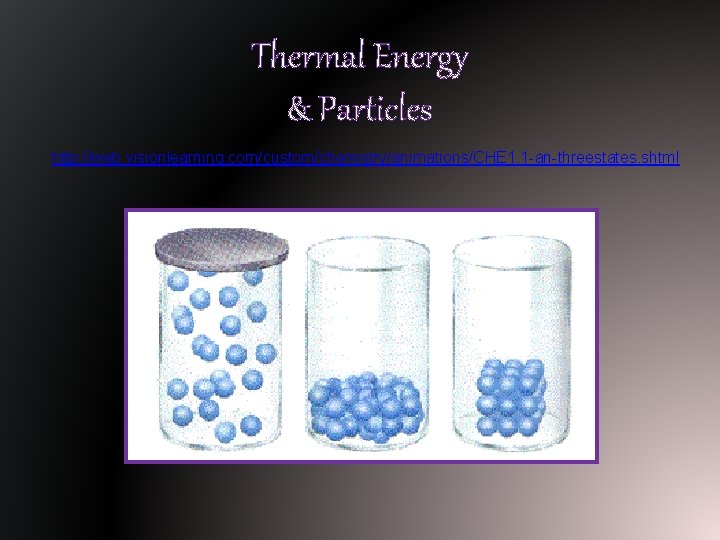
Thermal Energy & Particles http: //web. visionlearning. com/custom/chemistry/animations/CHE 1. 1 -an-threestates. shtml

A Fourth State? § If a gas is super heated, then it may change into a 4 th state of matter: Plasma. § The amount of thermal energy required to make matter a plasma is so high, atoms break apart into a mixture of negatively and positively charged particles.

A Fourth State? § Matter in its plasma state cannot exist permanently on earth. § When air (a gas) is struck by lightning, it temporarily turns plasmic while the bolt is coursing through it, but returns to its gas state after the lighting strike.

A Fourth State?

A Fourth State? § The gasses that fuel burning stars are constantly in their plasmic state. § The positive and negative particles shoot out of stars as radiation (gamma rays, radio waves, light, and heat are examples)

A Fourth State?
 Oo solid
Oo solid States of matter solid liquid gas
States of matter solid liquid gas Concept map of matter solid liquid and gas
Concept map of matter solid liquid and gas Thermal energy in states of matter
Thermal energy in states of matter Thermal energy in states of matter
Thermal energy in states of matter Uses of heat
Uses of heat Arrangement particles of liquid
Arrangement particles of liquid Section 3 using thermal energy
Section 3 using thermal energy Solid
Solid Plasma particles
Plasma particles Molecules of solid liquid and gas
Molecules of solid liquid and gas Metal non metal liquid venn diagram
Metal non metal liquid venn diagram The properties of solids liquids and gases
The properties of solids liquids and gases What state of matter is pepsi
What state of matter is pepsi S solid liquid or gas
S solid liquid or gas Why is gas easier to compress than liquid and solid
Why is gas easier to compress than liquid and solid Solid liquid gas examples
Solid liquid gas examples Solid liquid gas
Solid liquid gas Indefinite shape
Indefinite shape Physical properties of matter graphic organizer
Physical properties of matter graphic organizer Matter concept map
Matter concept map Gas liquid solid
Gas liquid solid Solid liquid gas
Solid liquid gas Solid liquid gas particles
Solid liquid gas particles Solid liquid gas difference
Solid liquid gas difference Gas liquid solid
Gas liquid solid Gas liquid solid
Gas liquid solid Ap chemistry solutions
Ap chemistry solutions Water expansion temperature graph
Water expansion temperature graph Solid gas liquid
Solid gas liquid