Thermal Energy and Heat Thermal energy is the









- Slides: 14

Thermal Energy and Heat

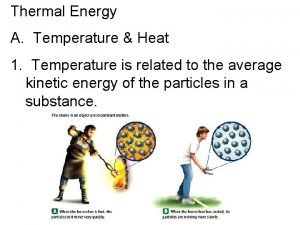
Thermal energy is the sum of the potential energy and the kinetic energy possessed by the molecules of an object. n n A hot object has more thermal energy than a cold object because the molecules are moving faster. A substance in its liquid state at its boiling point has less thermal energy (per kilogram) than when it is a gas at the same temperature. http: //phet. colorado. edu/sims/friction_en. html

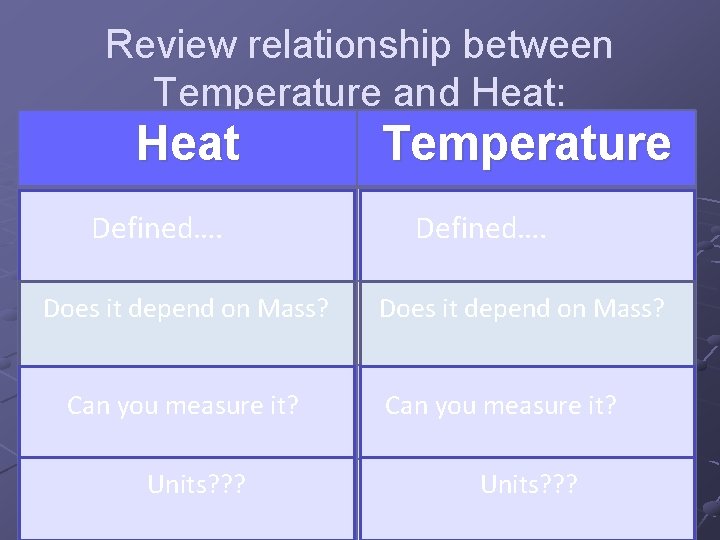
Review relationship between Temperature and Heat: Heat Temperature Thermal energy that is Defined…. transferred!! Measure of the average Defined…. Kinetic energy of the molecules in a substance! Does it depend on Mass? not mass Does it. Isdepend on Mass? Is mass dependent!! Cannot be measured directly!! Can you measure it? in Only by the changes temperature they cause!! dependent!! Can be youmeasuredirectly!! it? With a thermometer!! Units? ? ? Measured in o. C, o. F, or Measured in Joules(J) K Units? ? ?


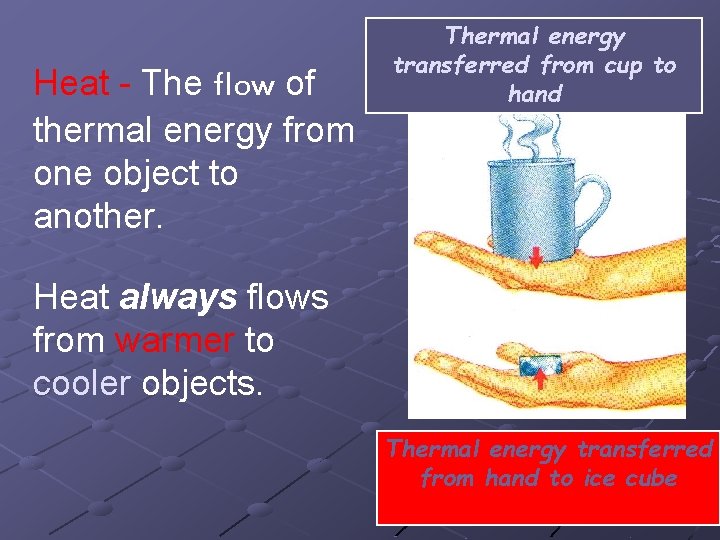
Heat - The flow of thermal energy from one object to another. Thermal energy transferred from cup to hand Heat always flows from warmer to cooler objects. Thermal energy transferred from hand to ice cube


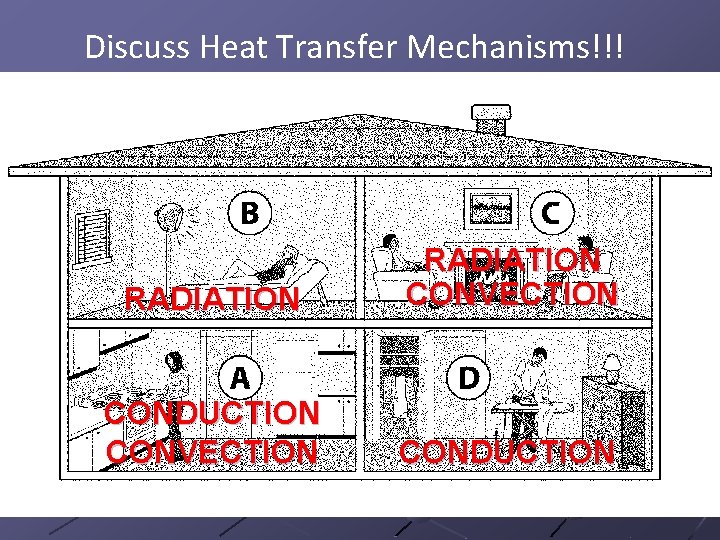
3 Ways to Transfer Heat 1. Conduction---direct contact. 2. Convection—transfer of heat in liquids and gases!! 3. Radiation—transfer through empty space!!



Discuss Heat Transfer Mechanisms!!! RADIATION CONVECTION CONDUCTION



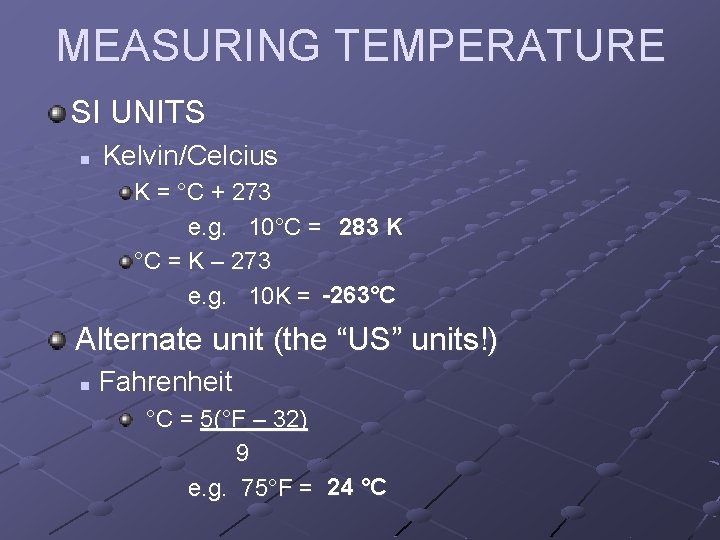
MEASURING TEMPERATURE SI UNITS n Kelvin/Celcius K = °C + 273 e. g. 10°C = 283 K °C = K – 273 e. g. 10 K = -263°C Alternate unit (the “US” units!) n Fahrenheit °C = 5(°F – 32) 9 e. g. 75°F = 24 °C

 Section 3 using thermal energy worksheet answer key
Section 3 using thermal energy worksheet answer key How are thermal energy and temperature different
How are thermal energy and temperature different What is the difference between thermal energy and heat?
What is the difference between thermal energy and heat? Difference between heat and thermal energy
Difference between heat and thermal energy Difference between heat and thermal energy
Difference between heat and thermal energy What is heat energy?
What is heat energy? What is the difference between thermal energy and heat?
What is the difference between thermal energy and heat? Heat thermal energy and temperature
Heat thermal energy and temperature Heat thermal energy and temperature
Heat thermal energy and temperature Chapter 16 thermal energy and heat
Chapter 16 thermal energy and heat Energy and temperature
Energy and temperature Heat vs thermal energy
Heat vs thermal energy Thermal energy vs heat
Thermal energy vs heat Heat vs thermal energy vs temperature
Heat vs thermal energy vs temperature Thermal energy vs temperature
Thermal energy vs temperature