Thermal Energy Heat and Its Uses THERMAL ENERGY











- Slides: 23

Thermal Energy & Heat and Its Uses

THERMAL ENERGY & MATTER Think About this… 1. 2. 3. 4. 5. 6. In which direction does heat flow spontaneously? Define TEMPERATURE How is THERMAL ENERGY transferred? What are the factors that determine the THERMAL ENERGY of a material? Which type of material heats more, one with a high specific heat, or one with a low specific heat? Is WORK 100% efficient? How do you know?

THERMAL ENERGY & MATTER l Work and Heat- work is never 100% efficient. Some is always lost to heat.

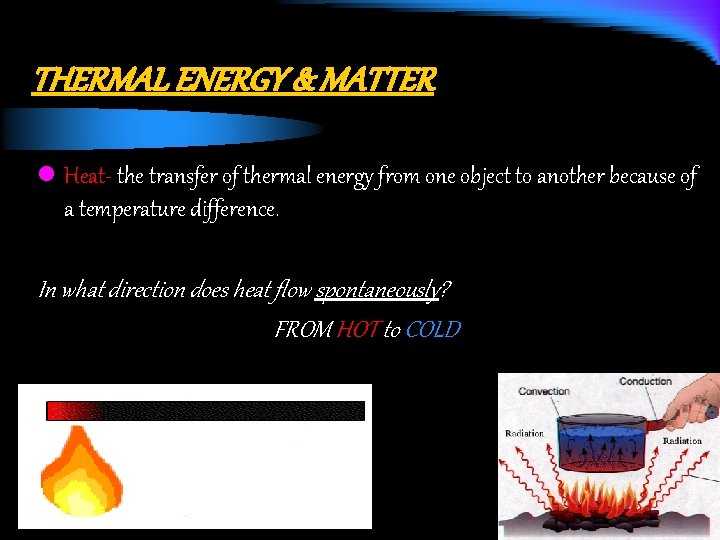
THERMAL ENERGY & MATTER l Heat- the transfer of thermal energy from one object to another because of a temperature difference. In what direction does heat flow spontaneously? FROM HOT to COLD

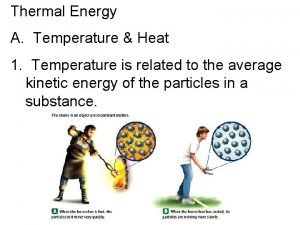
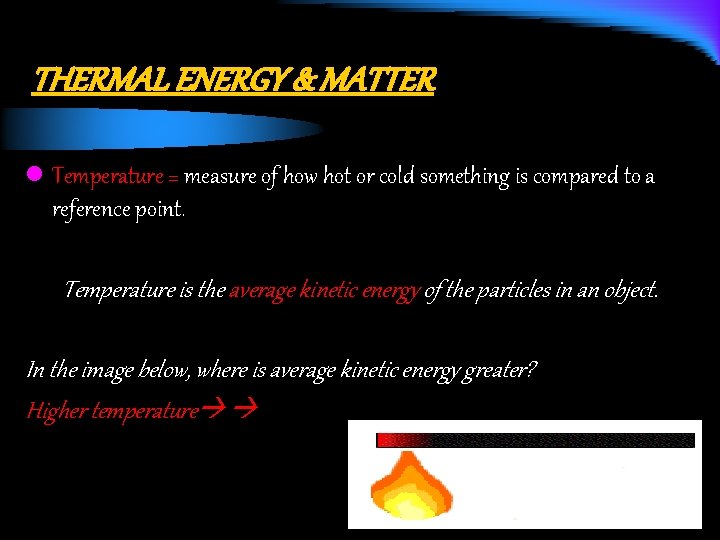
THERMAL ENERGY & MATTER l Temperature = measure of how hot or cold something is compared to a reference point. Temperature is the average kinetic energy of the particles in an object. In the image below, where is average kinetic energy greater? Higher temperature

THERMAL ENERGY & MATTER l Heat flows DOWN the bar through COLLISIONS. l Collisions transfer thermal energy from hot to cold.

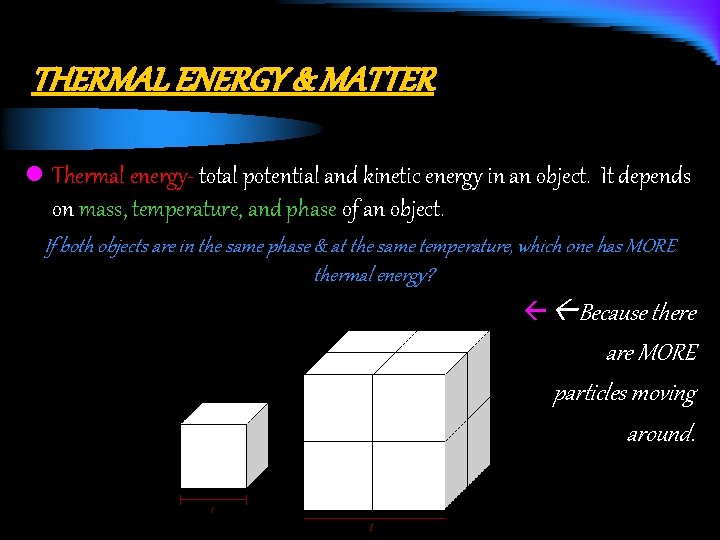
THERMAL ENERGY & MATTER l Thermal energy- total potential and kinetic energy in an object. It depends on mass, temperature, and phase of an object. If both objects are in the same phase & at the same temperature, which one has MORE thermal energy? ß Because there are MORE particles moving around.

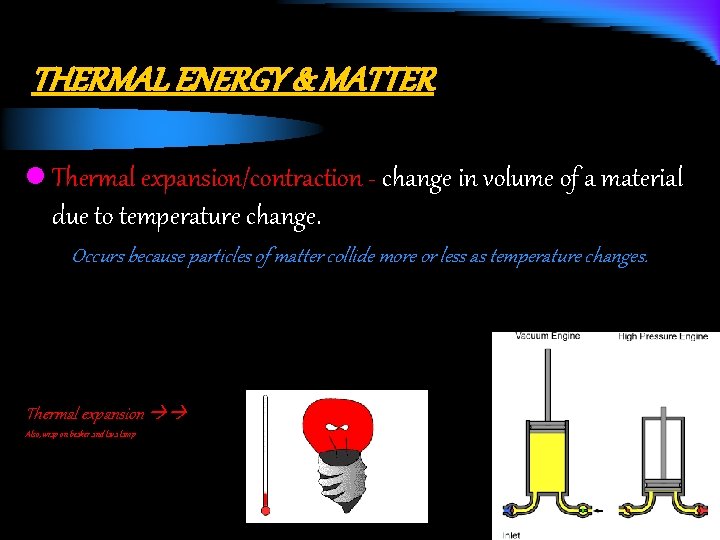
THERMAL ENERGY & MATTER l Thermal expansion/contraction - change in volume of a material due to temperature change. Occurs because particles of matter collide more or less as temperature changes. Thermal expansion Also, wrap on beaker and lava lamp

THERMAL ENERGY & MATTER l Specific Heat – amount of heat needed to raise ONE gram of a material ONE degree Celsius.

THERMAL ENERGY & MATTER l The LOWER a material’s specific heat the MORE its temperature rises when energy is added. Which will heat faster (has the lower specific heat)? Water? Specific heat of water = 4. 18 J/g°C Or Lead? YES! Specific heat of lead = 0. 46 J/g°C


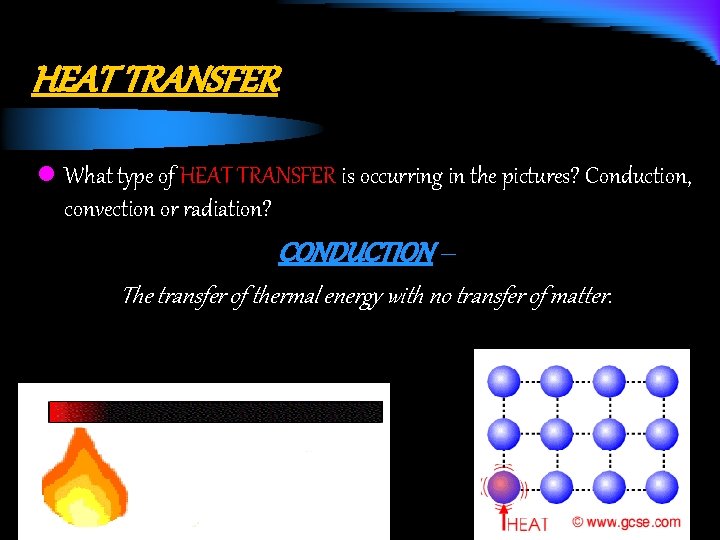
HEAT TRANSFER l What type of HEAT TRANSFER is occurring in the pictures? Conduction, convection or radiation? CONDUCTION – The transfer of thermal energy with no transfer of matter.

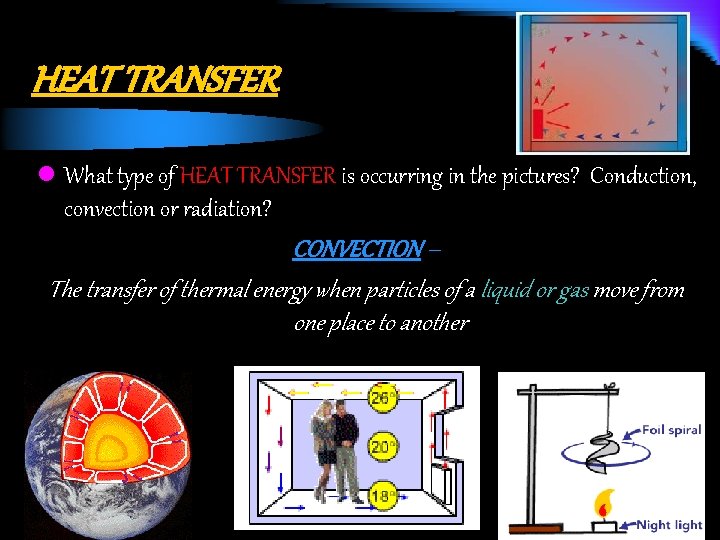
HEAT TRANSFER l What type of HEAT TRANSFER is occurring in the pictures? Conduction, convection or radiation? CONVECTION – The transfer of thermal energy when particles of a liquid or gas move from one place to another

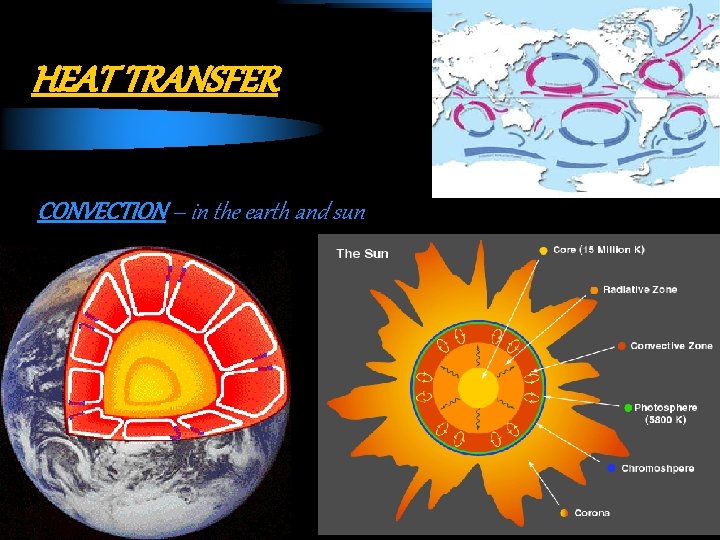
HEAT TRANSFER CONVECTION – in the earth and sun

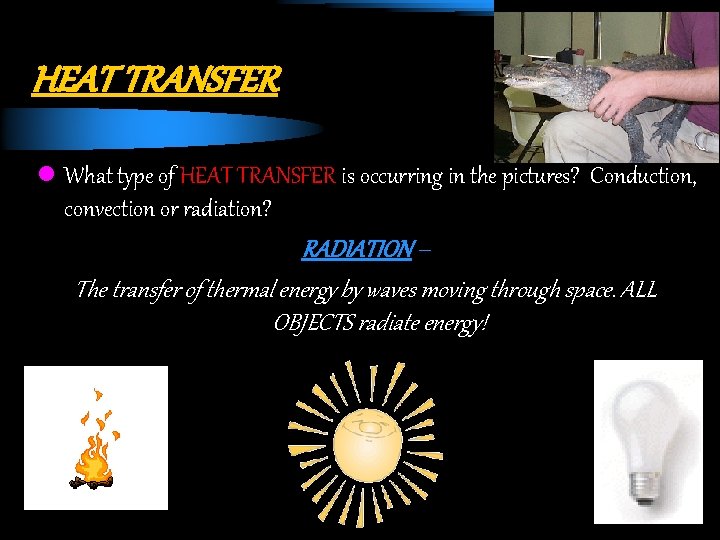
HEAT TRANSFER l What type of HEAT TRANSFER is occurring in the pictures? Conduction, convection or radiation? RADIATION – The transfer of thermal energy by waves moving through space. ALL OBJECTS radiate energy!

THERMODYNAMICS l The study of conversions between thermal energy and other forms of energy.

THERMODYNAMICS l First Law: Energy is Conserved


THERMAL ENERGY & MATTER Think about this… Define Convection, Conduction and Radiation 2. Give an example of each. 3. Write a sentence describing how each is important to our everyday lives. 4. How do we use heat in our everyday lives? 1.

Temperature Scales l Three different ones get used l Fahrenheit- the one we use l Celsius- metric standard l Kelvin- starts at absolute zero but same degree size as Celsius

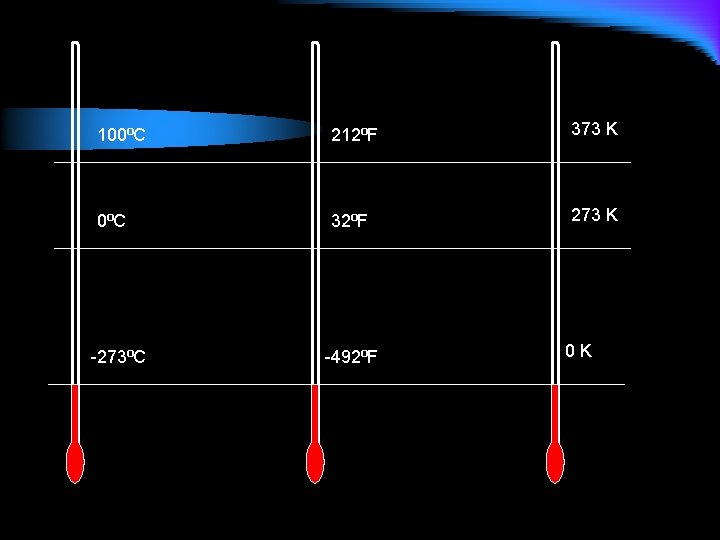
Converting Temperature l F = 9 C + 32 5 l K = C + 273 l Water freezes at 32°F, what is this in Celsius? l In Kevin? l Water boils at 100°C. What is this in Fahrenheit? l In Kelvin?

100⁰C 212⁰F 373 K 0⁰C 32⁰F 273 K -273⁰C -492⁰F 0 K

Conversion Practice l Body temperature is 98. 6°F, what is this in Celsius? In Kelvin? l Methanol boils at 75°C, what is this in Fahrenheit? , in Kelvin? l Lead melts at 600 K, what is this in Celsius? In Fahrenheit?
 Thermal energy section 3
Thermal energy section 3 How are thermal energy and temperature different
How are thermal energy and temperature different What is the difference between thermal energy and heat?
What is the difference between thermal energy and heat? Difference between heat and thermal energy
Difference between heat and thermal energy Difference between heat and thermal energy
Difference between heat and thermal energy Thermal energy vs heat
Thermal energy vs heat Flannel shirt conductor or insulator
Flannel shirt conductor or insulator Heat thermal energy and temperature
Heat thermal energy and temperature Heat flow
Heat flow Chapter 16 thermal energy and heat
Chapter 16 thermal energy and heat Heat vs thermal energy
Heat vs thermal energy Example of heat energy
Example of heat energy Thermal vs heat energy
Thermal vs heat energy Thermal energy vs heat
Thermal energy vs heat Q=mct
Q=mct Mass and thermal energy
Mass and thermal energy Thermal transfer vs direct thermal printing
Thermal transfer vs direct thermal printing Specific latent heat
Specific latent heat Lyophilic colloids
Lyophilic colloids Coconut tree and its uses
Coconut tree and its uses Bright filled paperweight
Bright filled paperweight Its halloween its halloween the moon is full and bright
Its halloween its halloween the moon is full and bright Thermal
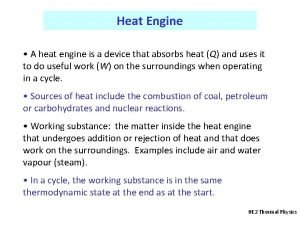
Thermal A heat engine is a device that uses
A heat engine is a device that uses