CHEMISTRY PROPERTIES OF MATTER CLASSIFYING MATTER v Matter






















- Slides: 37

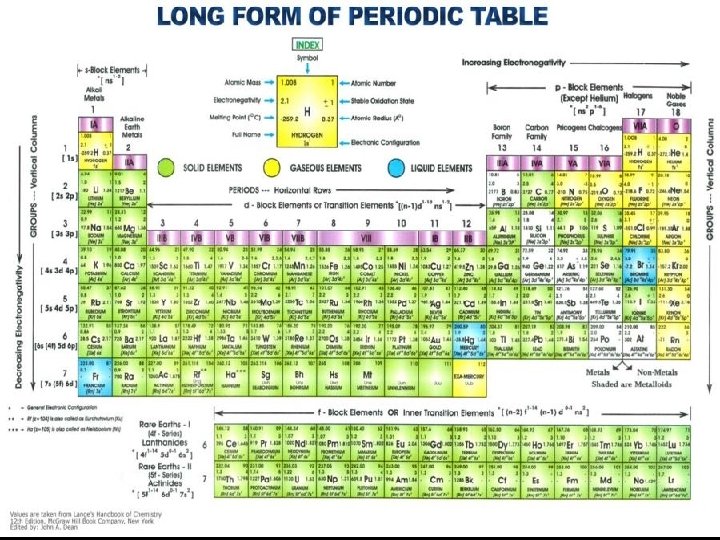
CHEMISTRY PROPERTIES OF MATTER

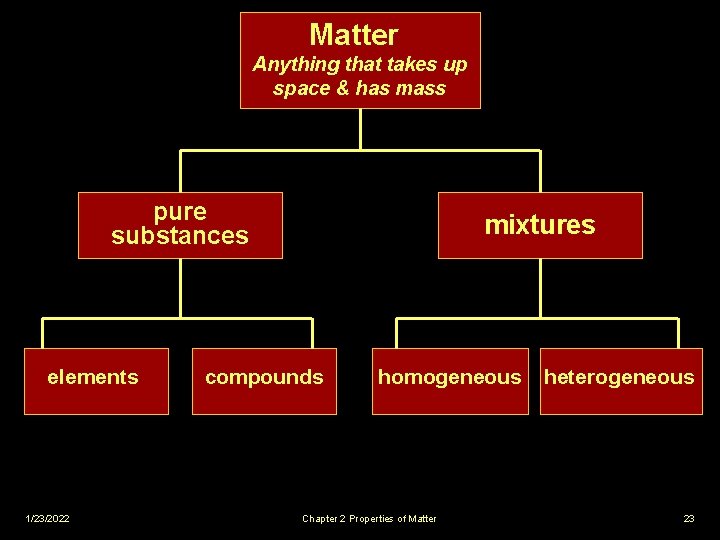
CLASSIFYING MATTER v Matter - Anything that takes up space and has mass v Based on their compositions, materials can be divided into pure substances and mixtures. 1/23/2022 Chapter 2 Properties of Matter 2

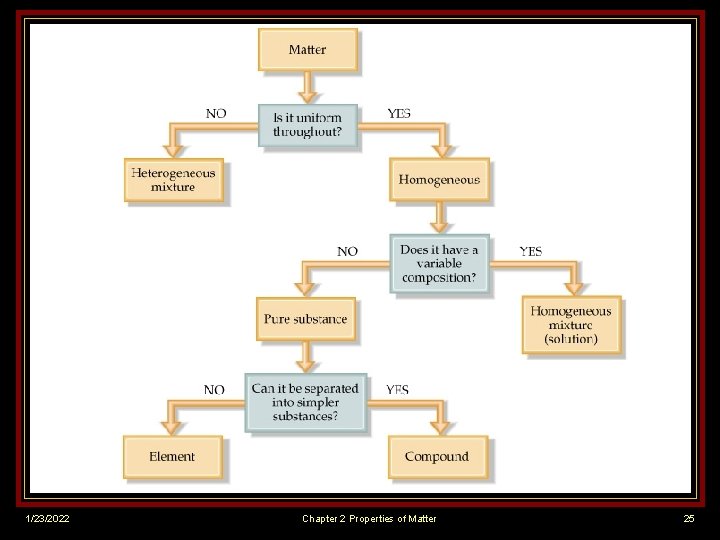
PURE SUBSTANCES (BOX 1) Pure substance (or simply a substance) matter that always has exactly the same composition. v Every sample has same properties. v Ex. Salt (Na. Cl); Pure Gold (Au) v v Can be classified into 2 categories: elements and compounds 1/23/2022 Chapter 2 Properties of Matter 3

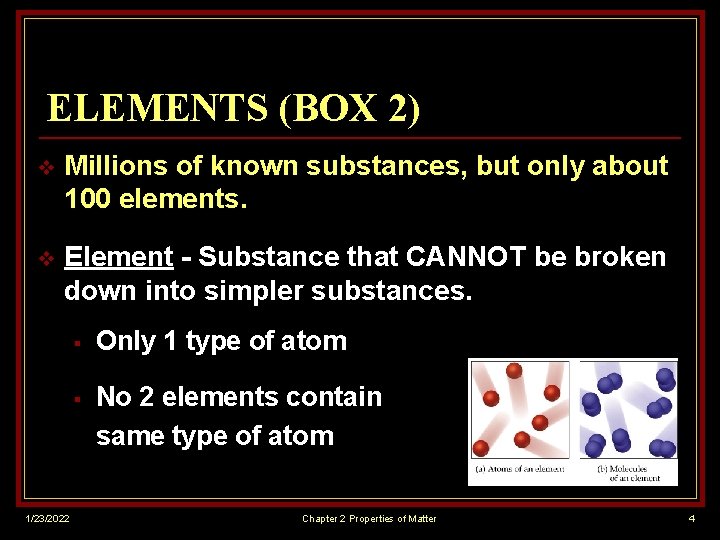
ELEMENTS (BOX 2) v Millions of known substances, but only about 100 elements. v Element - Substance that CANNOT be broken down into simpler substances. 1/23/2022 § Only 1 type of atom § No 2 elements contain same type of atom Chapter 2 Properties of Matter 4

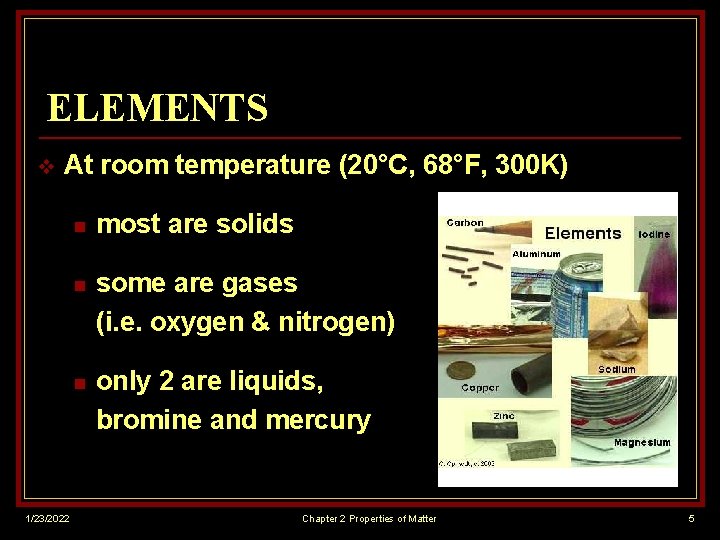
ELEMENTS v At room temperature (20°C, 68°F, 300 K) 1/23/2022 n most are solids n some are gases (i. e. oxygen & nitrogen) n only 2 are liquids, bromine and mercury Chapter 2 Properties of Matter 5

ELEMENTS v 1813, Jons Berzelius suggested use of symbols to represent elements. v Helps scientists to communicate without confusion 1/23/2022 Chapter 2 Properties of Matter 6

ELEMENTS 1 or 2 letters: 1 st capitalized, 2 nd not Ex. Oxygen (O); Chlorine (Cl) v v Some based on Latin names n v Symbol for gold is Au because Latin name for gold is aurum Sometimes name gives clue to properties n 1/23/2022 hydrogen from Greek words hydro (water) and genes (forming) Chapter 2 Properties of Matter 7

1/23/2022 Chapter 2 Properties of Matter 8

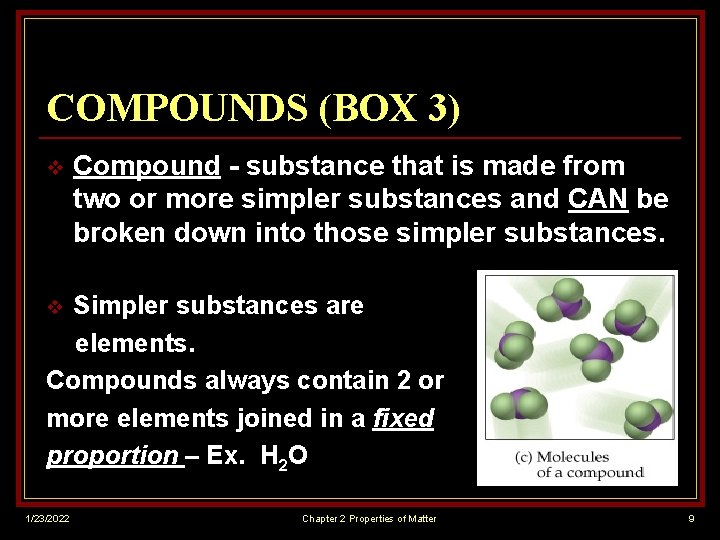
COMPOUNDS (BOX 3) v Compound - substance that is made from two or more simpler substances and CAN be broken down into those simpler substances. Simpler substances are elements. Compounds always contain 2 or more elements joined in a fixed proportion – Ex. H 2 O v 1/23/2022 Chapter 2 Properties of Matter 9

COMPOUNDS (BOX 3) v Always contains 2 or more elements joined in a fixed proportion. v Properties of a compound differ from those of the substances from which they are made. 1/23/2022 Chapter 2 Properties of Matter 10

COMPOUNDS (BOX 3) v 1/23/2022 Example: Water (H 2 O) Chapter 2 Properties of Matter 11

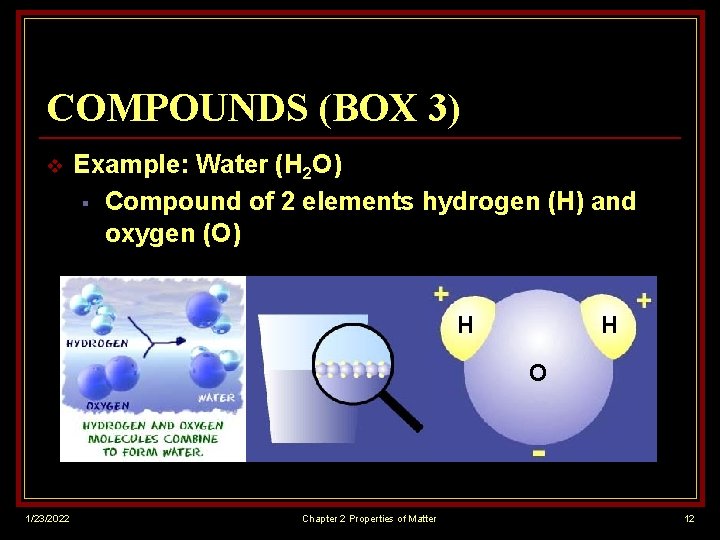
COMPOUNDS (BOX 3) v Example: Water (H 2 O) § Compound of 2 elements hydrogen (H) and oxygen (O) H H O 1/23/2022 Chapter 2 Properties of Matter 12

COMPOUNDS (BOX 3) v Example: Water (H 2 O) § H 2 & O 2 both gases at room temperature § H 2 can fuel a fire & O 2 can keep a fire burning § 1/23/2022 H 2 O does not burn or help other substances burn. Chapter 2 Properties of Matter Water decomposes into its component elements, hydrogen & oxygen, when direct electrical current is passed through it. Volume of hydrogen (right) is twice the volume of oxygen (left). 13

COMPOUNDS n Example: silicon dioxide (Si. O 2) n n 1/23/2022 Always 2 O atoms for each silicon (Si) atom n O colorless gas n Si gray solid Si. O 2 is a colorless, transparent solid Compound found in most light-colored grains of sand Chapter 2 Properties of Matter 14

MIXTURES - (Box 4) n n n 1/23/2022 Matter that doesn‘t always have the same composition. Mixtures NEVER become uniform. Tend to retain some of properties of their individual substances. Chapter 2 Properties of Matter 15

MIXTURES (Box 4) n 1/23/2022 The properties of a mixture can vary because the composition of a mixture is not fixed. Chapter 2 Properties of Matter 16

MIXTURES (Box 4) n No matter how well you stir, substances that make up mixture will not be evenly distributed. n Can be classified by how well parts are distributed throughout mixture. 1/23/2022 n Homogeneous n Heterogeneous Chapter 2 Properties of Matter 17

MIXTURES: HOMOGENEOUS (BOX 5) n Homogeneous Mixture - substances are so evenly mixed that it is difficult to see one substance in the mixture from another. n Appears to have only one type of substance. n Example: swimming pool water H 2 O + substances that dissolve in water Coffee n 1/23/2022 Chapter 2 Properties of Matter 18

MIXTURES: HETEROGENEOUS (BOX 6) n Greek words hetero (“different”) and genus (“kind”) Heterogeneous Mixture - parts of the mixture are noticeably different from one another. Example: Salad, Rocks and Sand n 1/23/2022 Chapter 2 Properties of Matter 19

MIXTURES: HOMOGENEOUS & HETEROGENEOUS 1/23/2022 Chapter 2 Properties of Matter 20

MIXTURES: HOMOGENEOUS Stainless steel serving spoon is a homogeneous mixture of iron, chromium, and nickel. It is difficult to distinguish one substance from another. 1/23/2022 Chapter 2 Properties of Matter 21

MIXTURES: HETEROGENEOUS Sand is a heterogeneous mixture. It is not the same throughout. 1/23/2022 Chapter 2 Properties of Matter 22

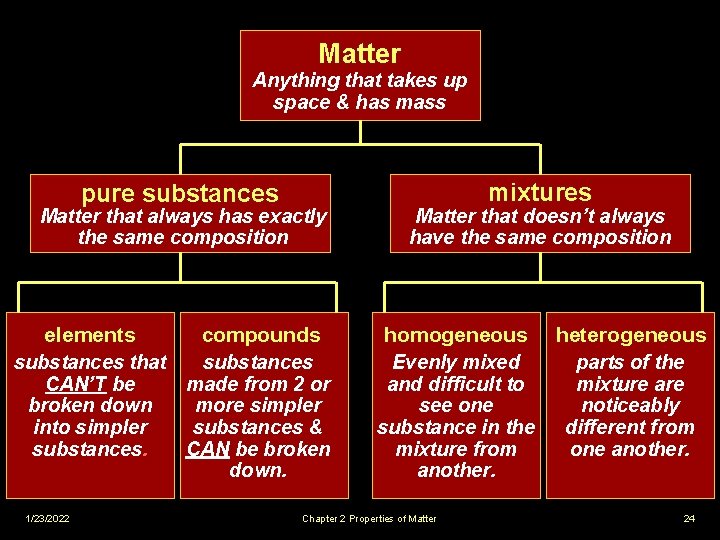
Matter Anything that takes up space & has mass pure substances elements 1/23/2022 mixtures compounds homogeneous Chapter 2 Properties of Matter heterogeneous 23

Matter Anything that takes up space & has mass pure substances Matter that always has exactly the same composition elements compounds substances that substances CAN’T be made from 2 or broken down more simpler into simpler substances & substances. CAN be broken down. 1/23/2022 mixtures Matter that doesn’t always have the same composition homogeneous Evenly mixed and difficult to see one substance in the mixture from another. Chapter 2 Properties of Matter heterogeneous parts of the mixture are noticeably different from one another. 24

1/23/2022 Chapter 2 Properties of Matter 25

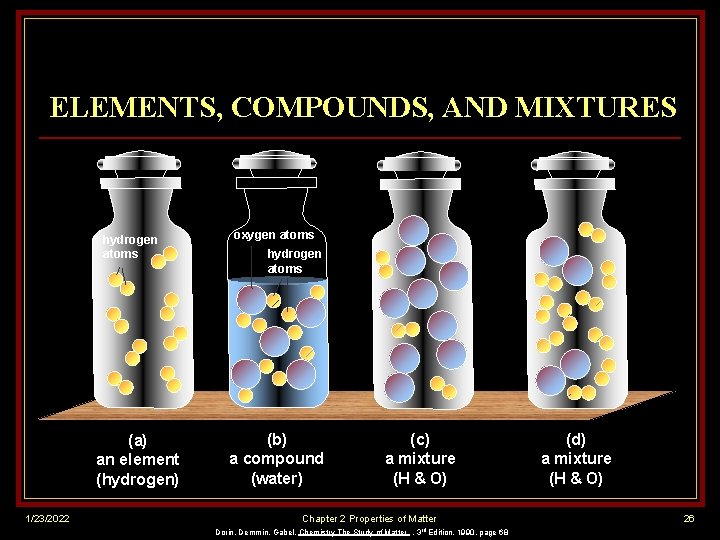
ELEMENTS, COMPOUNDS, AND MIXTURES hydrogen atoms (a) an element (hydrogen) 1/23/2022 oxygen atoms hydrogen atoms (b) a compound (water) (c) a mixture (H & O) Chapter 2 Properties of Matter Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 68 (d) a mixture (H & O) 26

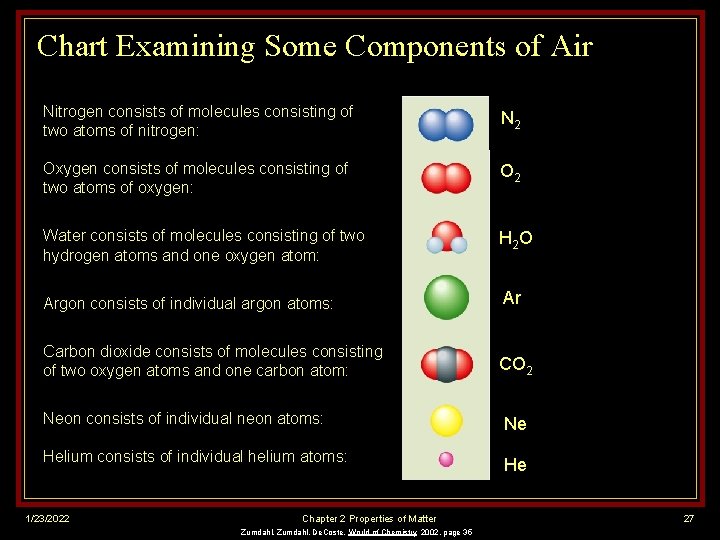
Chart Examining Some Components of Air Nitrogen consists of molecules consisting of two atoms of nitrogen: N 2 Oxygen consists of molecules consisting of two atoms of oxygen: O 2 Water consists of molecules consisting of two hydrogen atoms and one oxygen atom: H 2 O Argon consists of individual argon atoms: Ar Carbon dioxide consists of molecules consisting of two oxygen atoms and one carbon atom: CO 2 Neon consists of individual neon atoms: Ne Helium consists of individual helium atoms: He 1/23/2022 Chapter 2 Properties of Matter Zumdahl, De. Coste, World of Chemistry 2002, page 35 27

SOLUTIONS, SUSPENSIONS, AND COLLOIDS n The size of the particles in a mixture has an effect on the properties of that mixture. Key Concept: n Based on the size of its largest particles, a mixture can be classified as a solution, a suspension, or a colloid. 1/23/2022 Chapter 2 Properties of Matter 28

SOLUTIONS n Solution - mixture that forms when substances dissolve and form a homogeneous mixture n Example: Sugar dissolved in water Spoonful of sugar in a glass of hot water & stir, the sugar dissolves in the water homogeneous mixture of sugar & water. 1/23/2022 Chapter 2 Properties of Matter 29

SOLUTIONS n Properties of liquid solutions: Particles small so… n do not separate into distinct layers over time. n none of the substances in the solution are trapped in the filter. n can see through because light passes through them without being scattered in all directions. n Particles in a solution are too small to settle out of the solution, be trapped by a filter, or scatter light. 1/23/2022 Chapter 2 Properties of Matter 30

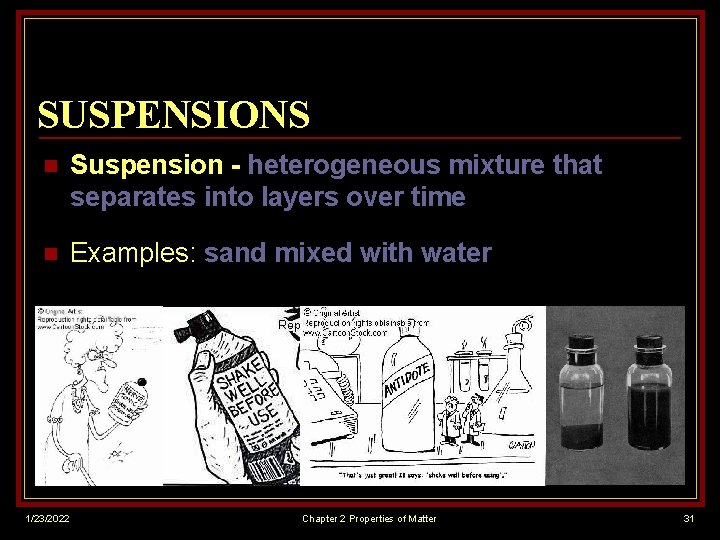
SUSPENSIONS n Suspension - heterogeneous mixture that separates into layers over time n Examples: sand mixed with water 1/23/2022 Chapter 2 Properties of Matter 31

SUSPENSIONS n 1/23/2022 Properties of a suspension: Because suspended particles are large… n suspended particles settle out of mixture (form layers) n can use a filter to separate out suspended particles n can scatter more light in all directions (cloudy) Chapter 2 Properties of Matter 32

COLLOIDS n Colloid - contains some particles that are intermediate in size between the small particles in a solution and the larger particles in a suspension n i. e. : homogenized milk, fog (water droplets in air) 1/23/2022 Chapter 2 Properties of Matter 33

COLLOIDS n 1/23/2022 Properties of a colloid: n does not separate into layers n can’t use a filter to separate the parts n scatters light (cloudy, opaque) Chapter 2 Properties of Matter 34

Key Concepts for Solutions, Suspensions & Colloids n 1/23/2022 Properties of liquid solutions: Particles small so… n Particles will not separate into layers over time. n None of the substances in the solution can be trapped in a filter. n Can see through the solution because light passes through them without being scattered in all directions. Chapter 2 Properties of Matter 35

Key Concepts for Solutions, Suspensions & Colloids n 1/23/2022 Properties of a suspension: Because suspended particles are large… n Suspended particles settle out of a mixture (Parts WILL separate). n CAN use a filter to separate out suspended particles. n Can scatter more light in all directions (cloudy) Chapter 2 Properties of Matter 36

Key Concepts for Solutions, Suspensions & Colloids n 1/23/2022 Properties of a colloid: n Does NOT separate into layers. n Can not use a filter to separate the parts. n Scatters light (cloudy, opaque). The light will be reflected by the larger particles in a colloid. Chapter 2 Properties of Matter 37
 Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Classifying matter quiz
Classifying matter quiz Flowchart for matter
Flowchart for matter Worksheet classification of matter
Worksheet classification of matter Matter is classified by
Matter is classified by Copper wire classification of matter
Copper wire classification of matter Classification of matter graphic organizer
Classification of matter graphic organizer Classification of matter flow chart
Classification of matter flow chart Classifying matter quiz
Classifying matter quiz Concept map properties of matter
Concept map properties of matter Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Atomic structure and properties ap chemistry
Atomic structure and properties ap chemistry Molarity equation examples
Molarity equation examples Ap chemistry chapter 7 periodic properties of the elements
Ap chemistry chapter 7 periodic properties of the elements Extensive vs intensive
Extensive vs intensive Chemical property of matter
Chemical property of matter Chemistry matter and its changes
Chemistry matter and its changes Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 chemistry study guide
Chapter 10 chemistry study guide Examples of matter in chemistry
Examples of matter in chemistry Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Chemistry matter and change chapter 2 answer key
Chemistry matter and change chapter 2 answer key Matter flowchart chemistry
Matter flowchart chemistry Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Foods that are heterogeneous mixtures
Foods that are heterogeneous mixtures Do
Do Properties of matter vocabulary
Properties of matter vocabulary Objectives of properties of matter
Objectives of properties of matter Common properties of matter
Common properties of matter Classification and properties of matter
Classification and properties of matter Properties and changes of matter worksheet
Properties and changes of matter worksheet Measurable properties of matter examples
Measurable properties of matter examples States of matter jeopardy
States of matter jeopardy Properties of matter jeopardy
Properties of matter jeopardy