UNIT 1 ATOMIC STRUCTURE AND PROPERTIES AP Chemistry





![TEMPERATURE CHANGES Celsius to Kelvin [°C] = [K] − 273. 15 Fahrenheit to Celsius TEMPERATURE CHANGES Celsius to Kelvin [°C] = [K] − 273. 15 Fahrenheit to Celsius](https://slidetodoc.com/presentation_image_h/2e8d2de15fdb851dae58db67505cb7a1/image-16.jpg)




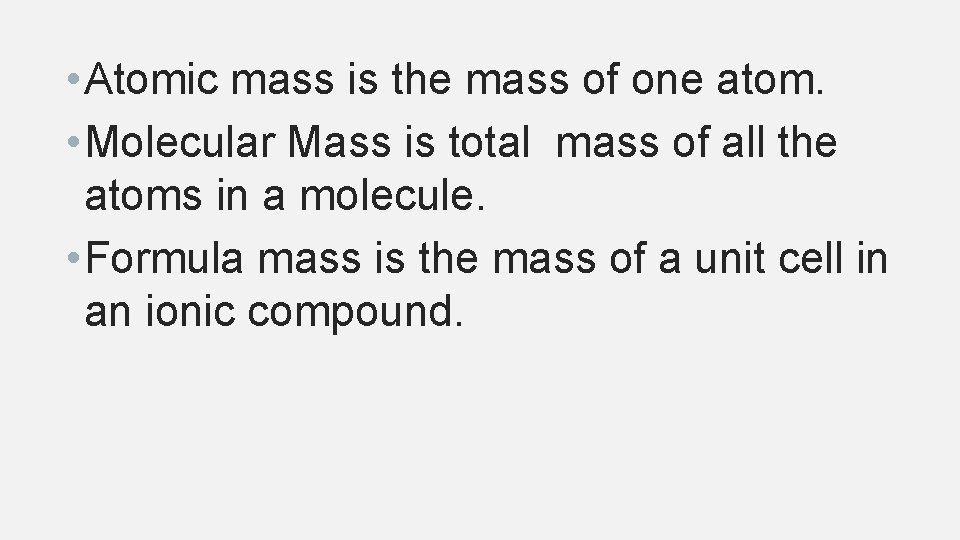


![MASS SPECTRUM OF CO 2 Molecular ion peak [CO 2]+ = 44 Fragment Peaks MASS SPECTRUM OF CO 2 Molecular ion peak [CO 2]+ = 44 Fragment Peaks](https://slidetodoc.com/presentation_image_h/2e8d2de15fdb851dae58db67505cb7a1/image-36.jpg)








![SOME ANOMALIES For instance, the electron configuration for copper is [Ar] 4 s 1 SOME ANOMALIES For instance, the electron configuration for copper is [Ar] 4 s 1](https://slidetodoc.com/presentation_image_h/2e8d2de15fdb851dae58db67505cb7a1/image-96.jpg)

- Slides: 100

UNIT 1: ATOMIC STRUCTURE AND PROPERTIES AP Chemistry Mr. Lamberth

C. E. R. R q. Claim: An inference or answer to a question or problem. q. Evidence: Scientific data that supports the claim. q. Reasoning: Justification that links the evidence to the claim. q. Rebuttal: Alternative explanations and counter evidence as to why these other explanations are not correct.

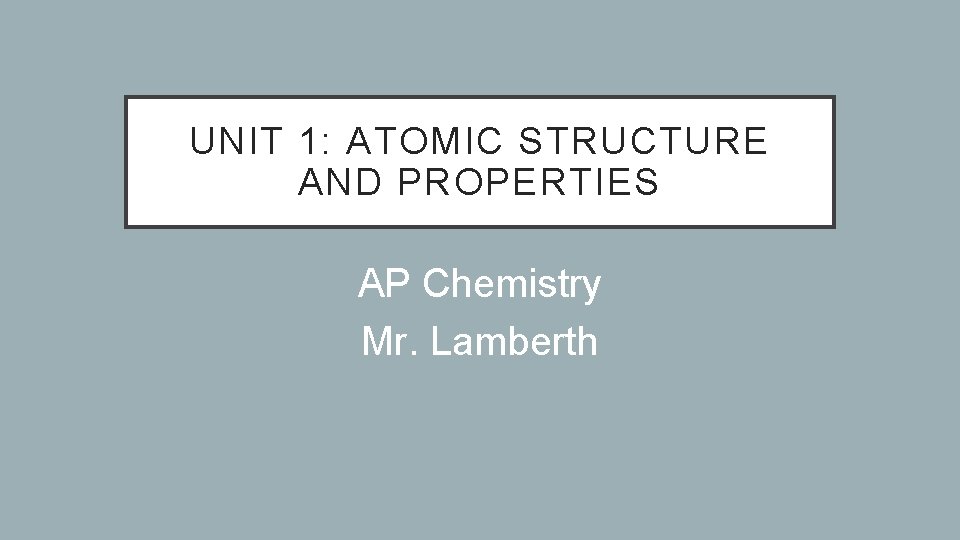
CERR EXAMPLE (PENNY LAB) Question Claim Evidence Reasoning Rebuttal What type of process took place (mixing, phase change, or chemical reaction)? A chemical reaction occurred. Before, the penny was brownish in color, was not soluble in water, and had a density of 8. 96 g/cm 3. After the experiment, the green solid was formed, soluble in water, and had a density of 1. 88 g/cm 3. The color, solubility, and density changed. Color, solubility, and density are all properties. Since the properties changed, I know a new substance was made, which means a chemical reaction occurred. Chemical reactions create new substances that have different properties from the old substances. Another explanation could be that a mixture was created or a third explanation could be that a phase change occurred. Since there is a new substance, it cannot be a mixture or a phase change. A mixture would just be a combination of the old substances and a phase change would be the same substance in a different state.

C. E. R. R Predict the next three letters in the series P N T L T ? ? ? q Claim: An inference or answer to a question or problem q Evidence: Scientific data that supports the claim q Reasoning: Justification that links the evidence to the claim q Rebuttal: Alternative explanations and counter evidence as to why these other explanations are not correct.

CLASSIFICATION OF MATTER AND MEASUREMENT REVIEW Review Questions: 1. 18, 1. 20, 1. 24, 1. 25, 1. 31, 1. 33, 1. 36, 1. 39, 1. 44, 1. 45, 1. 48, 1. 51, 1. 54, 1. 64, 1. 74

MATTER AND MEASUREMENT

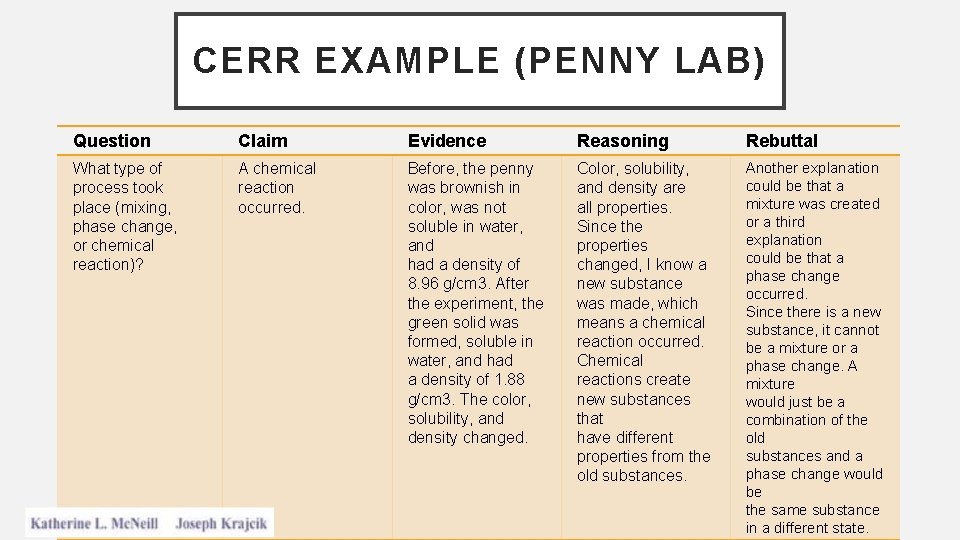
• Matter is anything that has mass and takes up space • Atom: The smallest possible unit of matter that still maintains an element's identity during chemical reactions • Molecule: two or more atoms bonded together with a covalent bond • If all the atoms bonded together are of the same type the molecule formed is still an element • If different types of atoms are bonded together, then the

Molecules of compounds Molecule of an element

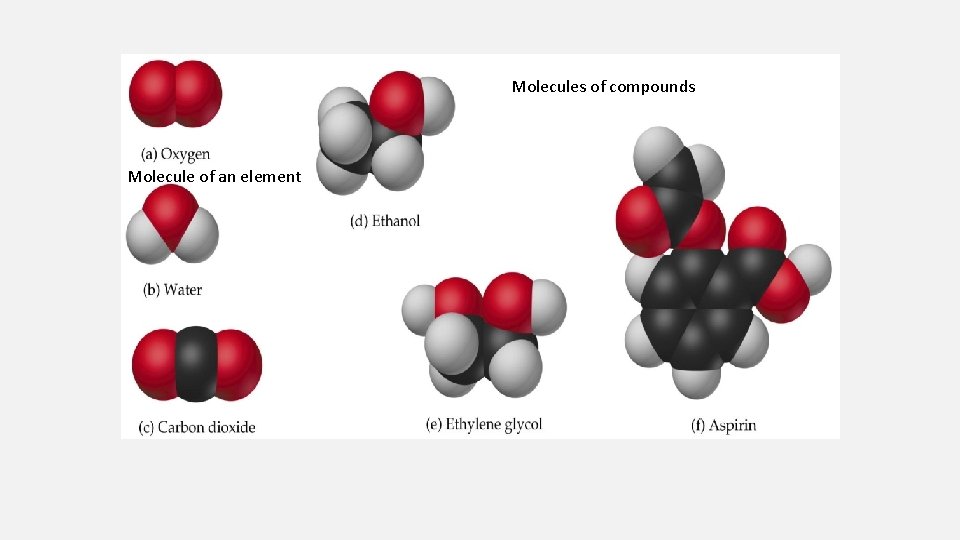
PHASES OF MATTER

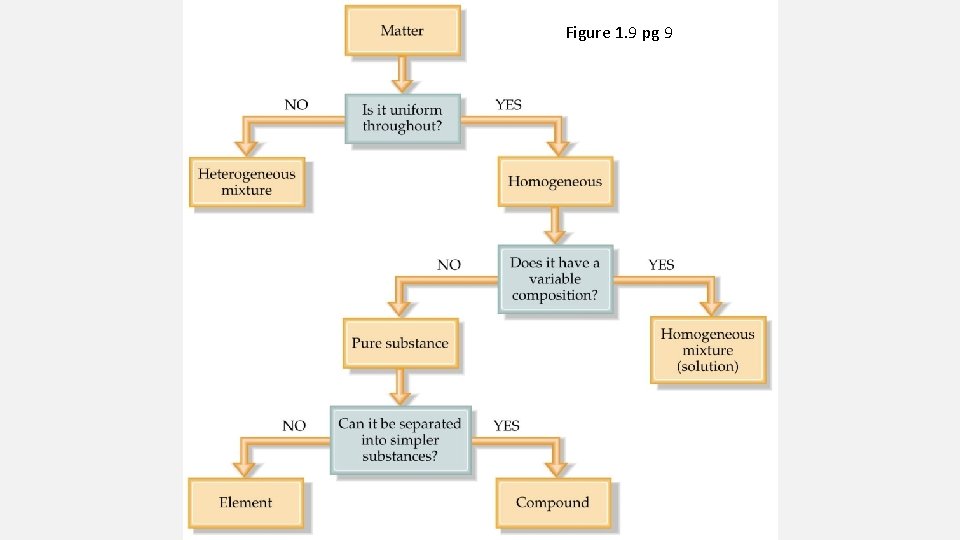
• Pure substances – • Elements - all the same type of atom. • Compounds - substances made from two or more different kinds of atoms. (follows law of constant composition – H 2 O) • Mixtures • Homogeneous - same throughout with identical properties everywhere in the mixture. This type of mixture is called a solution

Figure 1. 9 pg 9


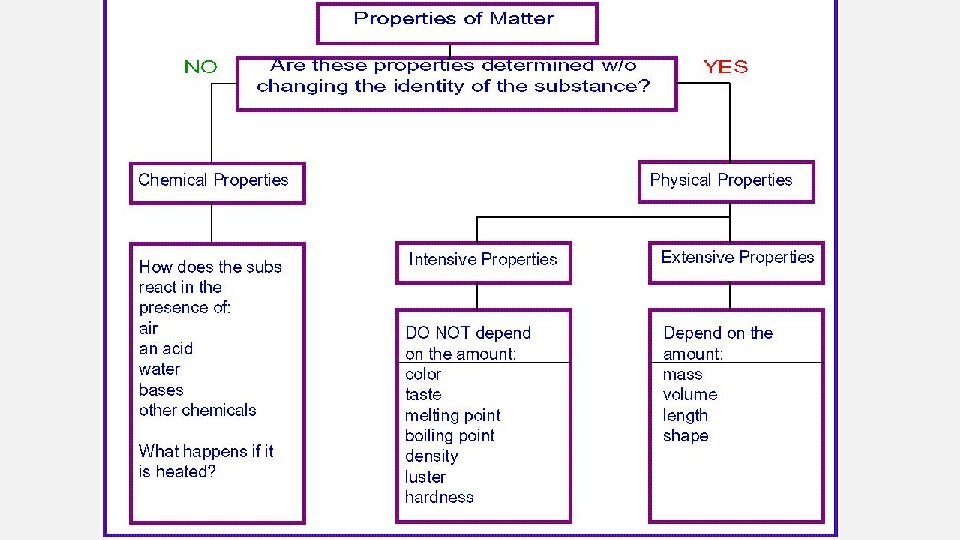
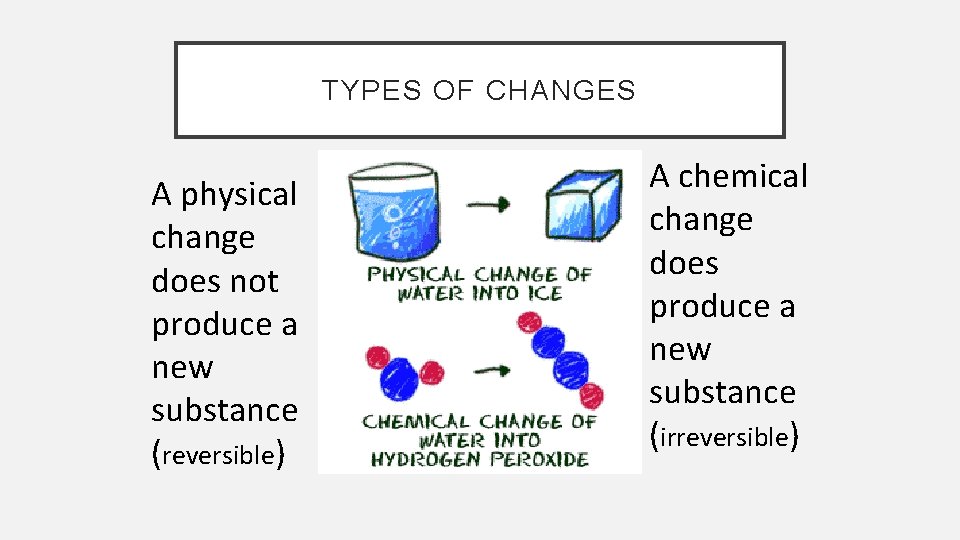
TYPES OF CHANGES A physical change does not produce a new substance (reversible) A chemical change does produce a new substance (irreversible)

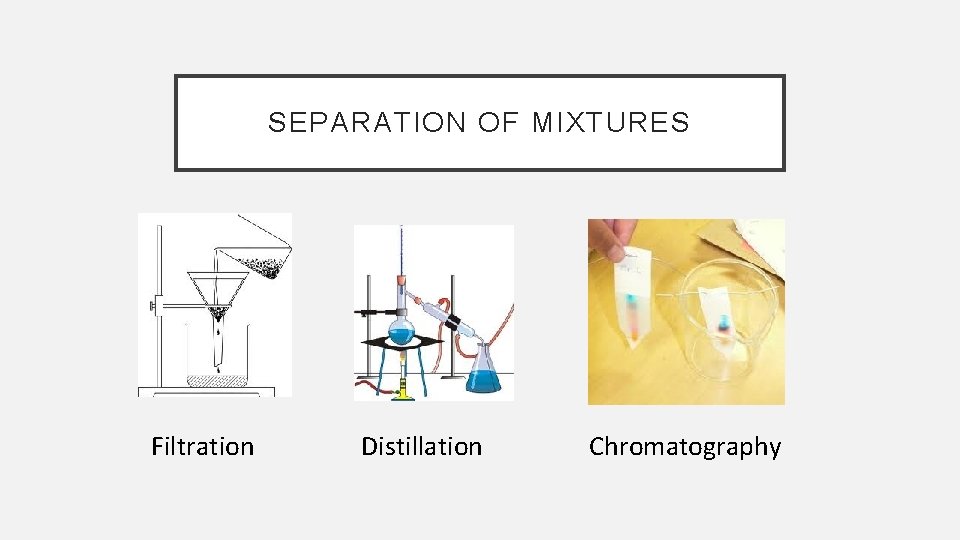
SEPARATION OF MIXTURES Filtration Distillation Chromatography

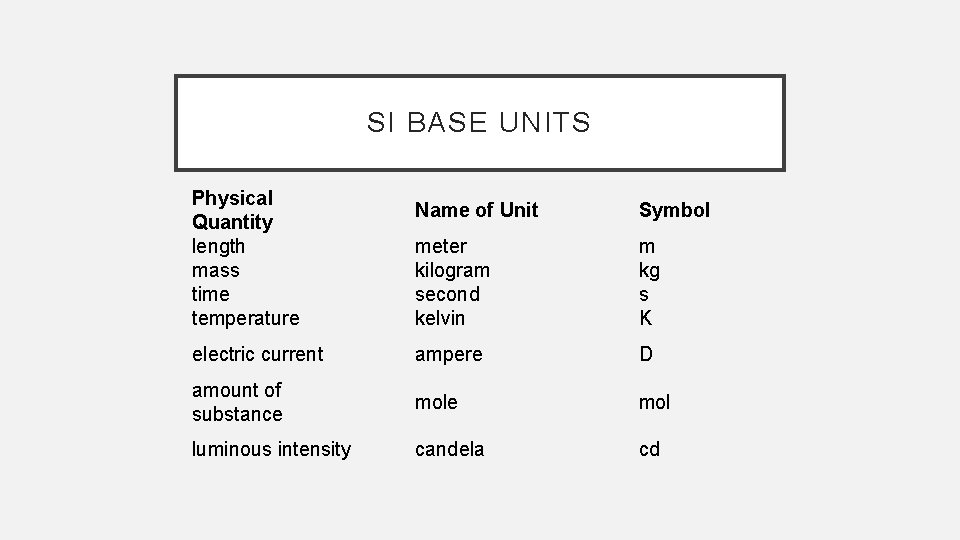
SI BASE UNITS Physical Quantity length mass time temperature Name of Unit Symbol meter kilogram second kelvin m kg s K electric current ampere D amount of substance mol luminous intensity candela cd
![TEMPERATURE CHANGES Celsius to Kelvin C K 273 15 Fahrenheit to Celsius TEMPERATURE CHANGES Celsius to Kelvin [°C] = [K] − 273. 15 Fahrenheit to Celsius](https://slidetodoc.com/presentation_image_h/2e8d2de15fdb851dae58db67505cb7a1/image-16.jpg)
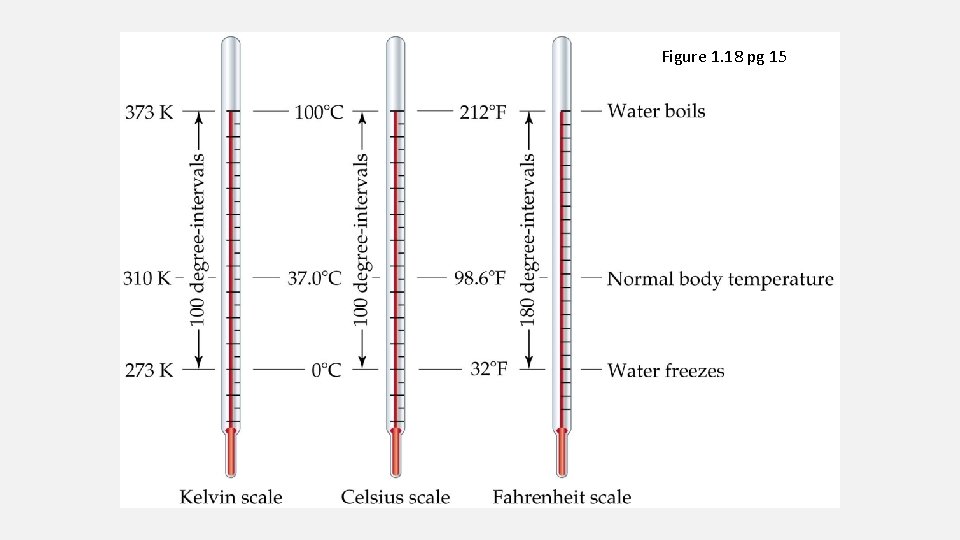
TEMPERATURE CHANGES Celsius to Kelvin [°C] = [K] − 273. 15 Fahrenheit to Celsius [°F] = [°C] × 9⁄5 + 32 Kelvin to Celsius [K] = [°C] + 273. 1 5 Celsius to Fahrenheit [°C] = ([°F] − 32) × 5⁄9

Figure 1. 18 pg 15

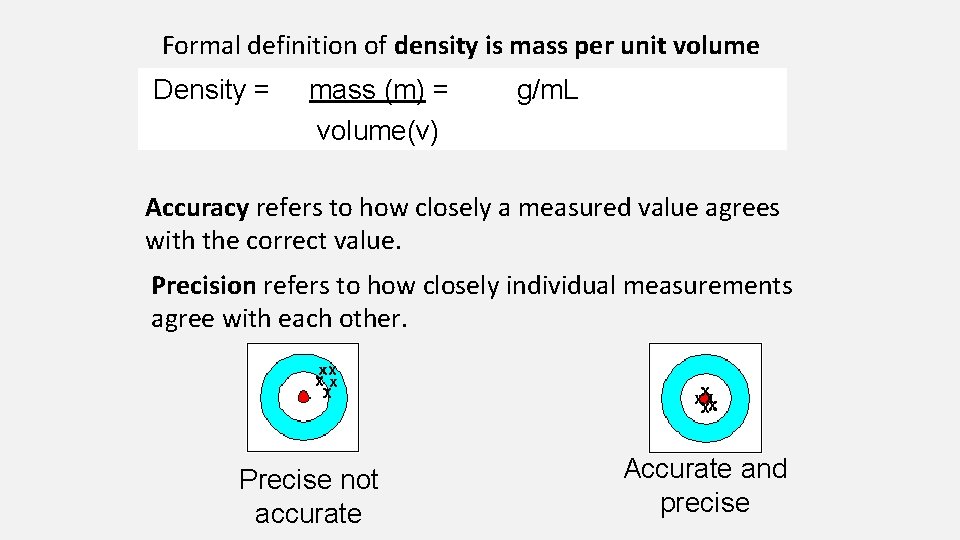
Formal definition of density Density = mass (m) = volume(v) is mass per unit volume g/m. L Accuracy refers to how closely a measured value agrees with the correct value. Precision refers to how closely individual measurements agree with each other. Precise not accurate Accurate and precise

Significant Figures • Non-zero numbers are always significant. • Zeros between non-zero numbers are always significant. • Zeros before the first non-zero digit are not significant. (Example: 0. 0003 has one significant figure. ) • Zeros at the end of the number after a decimal place are significant. • Zeros at the end of a number before a decimal place are ambiguous (e. g. 10, 300 g).

CALCULATING WITH SIG FIGS • When adding and subtracting round answer to the number with the least number of decimal places • When multiplying and dividing round answer to the number

DO THESE • Review Questions: 1. 18, 1. 20, 1. 24, 1. 25, 1. 31, 1. 33, 1. 36, 1. 39, 1. 44, 1. 45, 1. 48, 1. 51, 1. 54, 1. 64, 1. 74

1. 1: MOLE AND MOLAR MASS BL: 3. 4 Questions: 3. 28, 3. 32

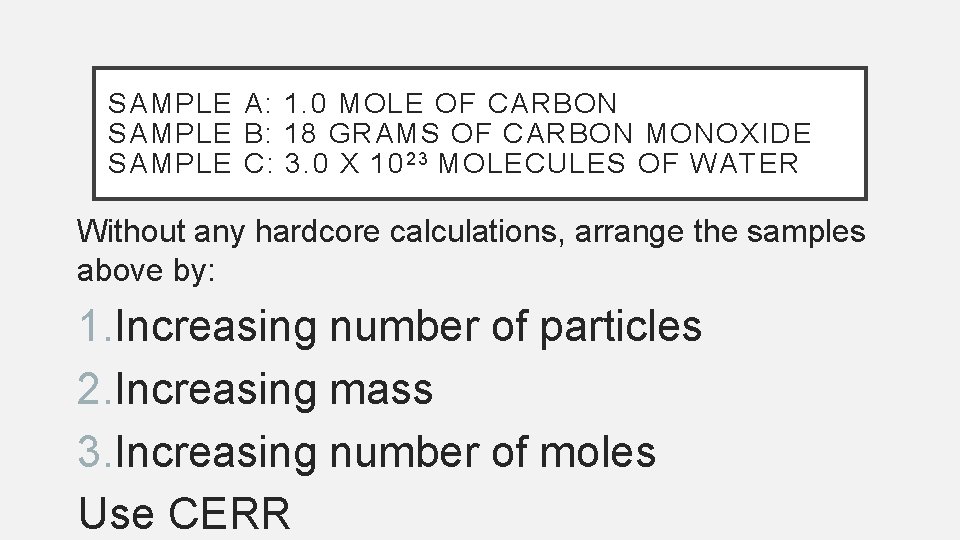
SAMPLE A: 1. 0 MOLE OF CARBON SAMPLE B: 18 GRAMS OF CARBON MONOXIDE SAMPLE C: 3. 0 X 10 2 3 MOLECULES OF WATER Without any hardcore calculations, arrange the samples above by: 1. Increasing number of particles 2. Increasing mass 3. Increasing number of moles Use CERR

ANSWERS • # particles: C, B, A or A, B, C Why? • Mass: C, A, B • Mole: C, B, A

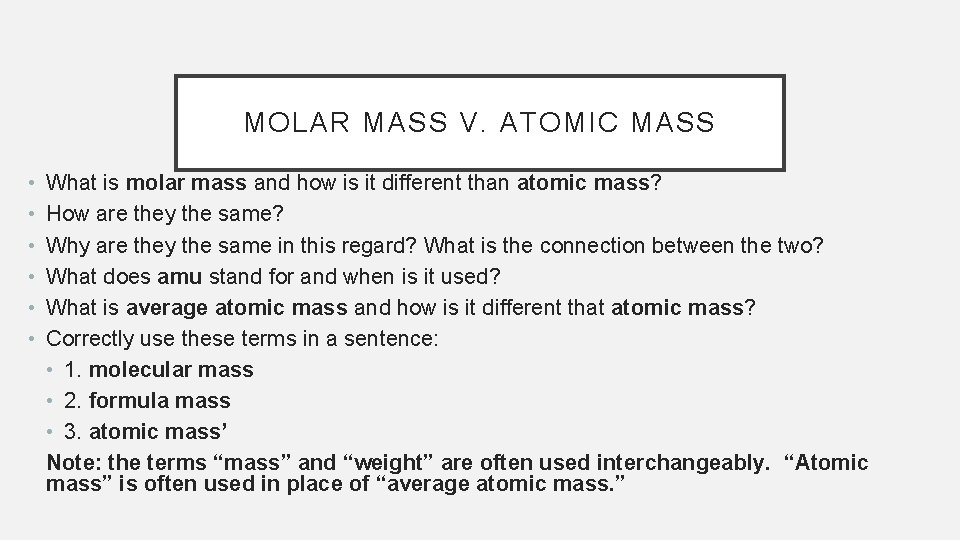
MOLAR MASS V. ATOMIC MASS • • • What is molar mass and how is it different than atomic mass? How are they the same? Why are they the same in this regard? What is the connection between the two? What does amu stand for and when is it used? What is average atomic mass and how is it different that atomic mass? Correctly use these terms in a sentence: • 1. molecular mass • 2. formula mass • 3. atomic mass’ Note: the terms “mass” and “weight” are often used interchangeably. “Atomic mass” is often used in place of “average atomic mass. ”
• Atomic mass is the mass of one atom. • Molecular Mass is total mass of all the atoms in a molecule. • Formula mass is the mass of a unit cell in an ionic compound.

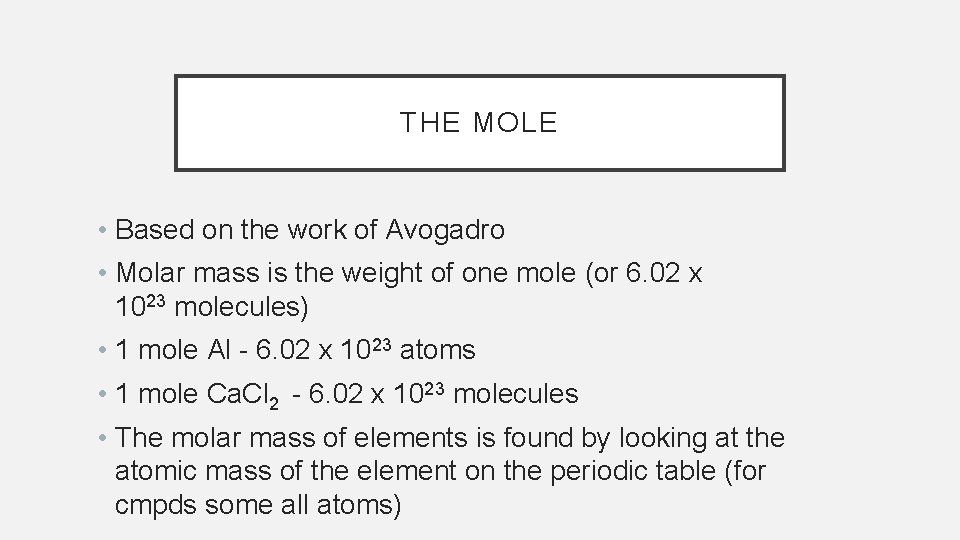
THE MOLE • Based on the work of Avogadro • Molar mass is the weight of one mole (or 6. 02 x 1023 molecules) • 1 mole Al - 6. 02 x 1023 atoms • 1 mole Ca. Cl 2 - 6. 02 x 1023 molecules • The molar mass of elements is found by looking at the atomic mass of the element on the periodic table (for cmpds some all atoms)

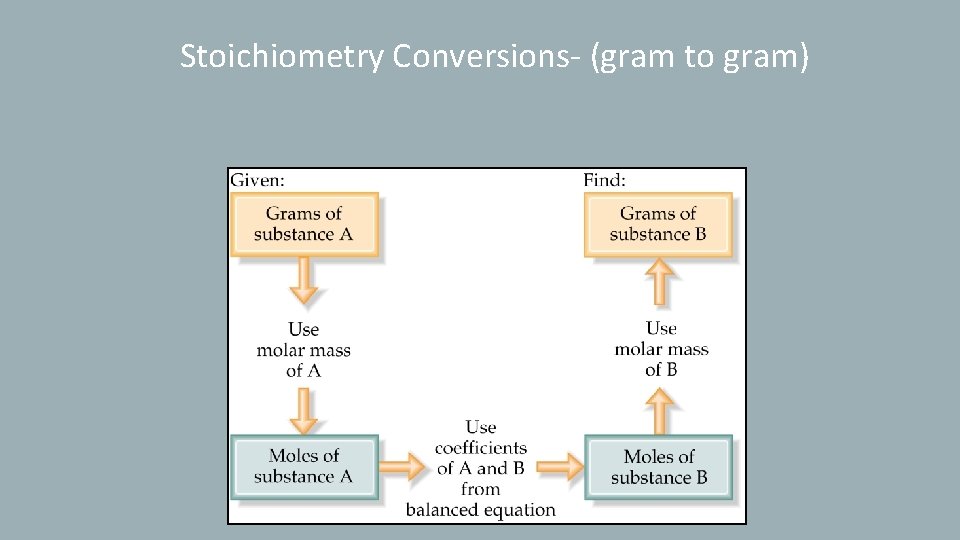
Stoichiometry Conversions- (gram to gram)

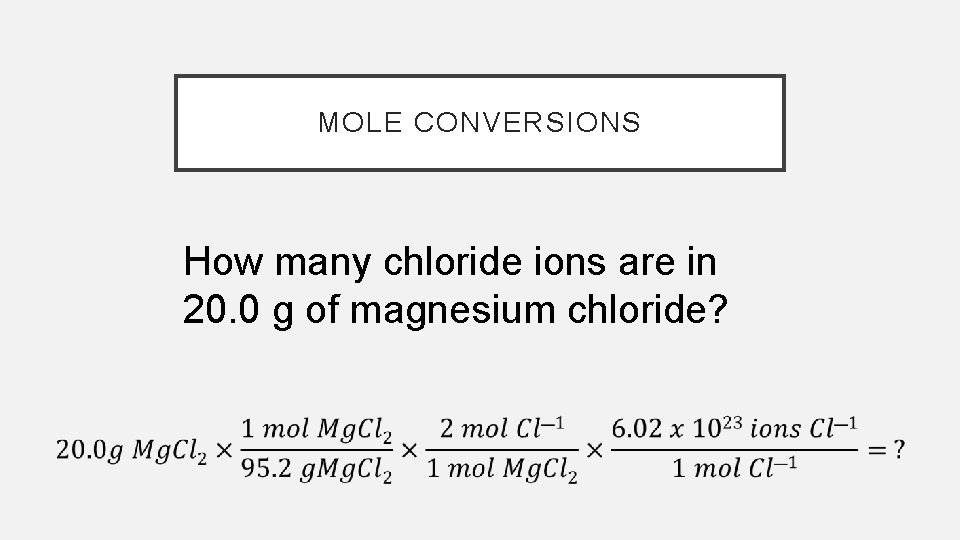
MOLE CONVERSIONS How many chloride ions are in 20. 0 g of magnesium chloride?

DO THESE • Review Questions: 1. 18, 1. 20, 1. 24, 1. 25, 1. 31, 1. 33, 1. 36, 1. 39, 1. 44, 1. 45, 1. 48, 1. 51, 1. 54, 1. 64, 1. 74 • Questions: 3. 28, 3. 32

1. 2 MASS SPECTROSCOPY OF ELEMENTS BL 2. 4 (Look at A Closer Look on p. 45) Questions: 2. 24, 2. 26, 2. 28

MASS SPECTROMETRY Courtesy www. lab-initio. com

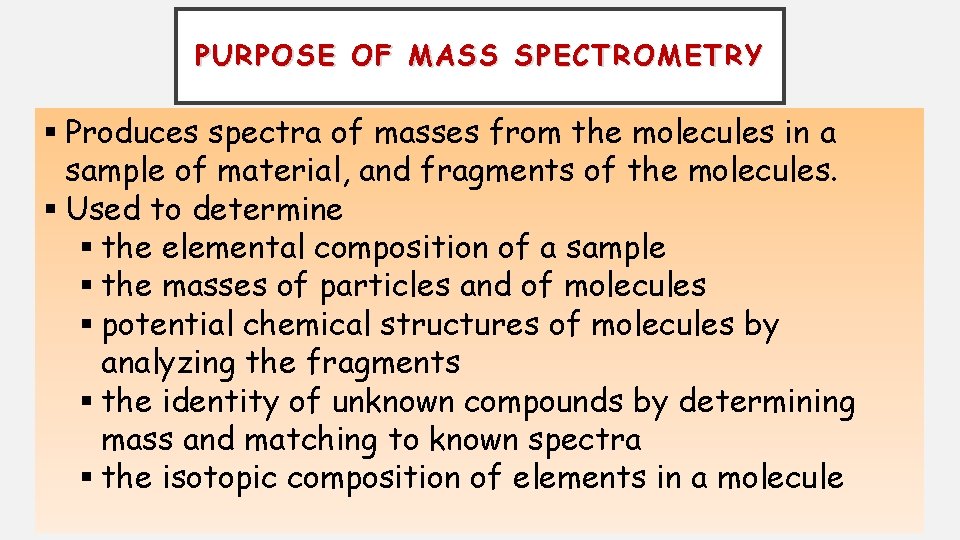
PURPOSE OF MASS SPECTROMETRY § Produces spectra of masses from the molecules in a sample of material, and fragments of the molecules. § Used to determine § the elemental composition of a sample § the masses of particles and of molecules § potential chemical structures of molecules by analyzing the fragments § the identity of unknown compounds by determining mass and matching to known spectra § the isotopic composition of elements in a molecule

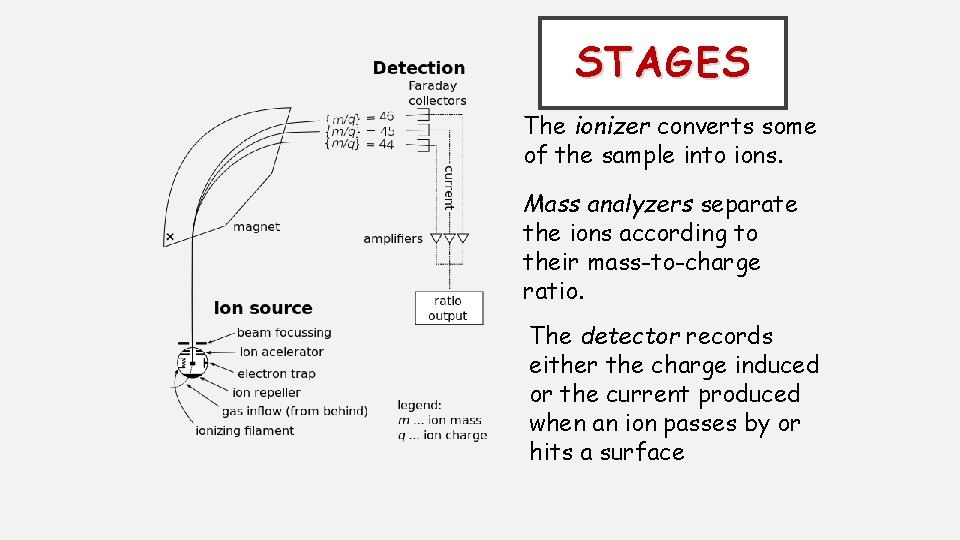
STAGES The ionizer converts some of the sample into ions. Mass analyzers separate the ions according to their mass-to-charge ratio. The detector records either the charge induced or the current produced when an ion passes by or hits a surface

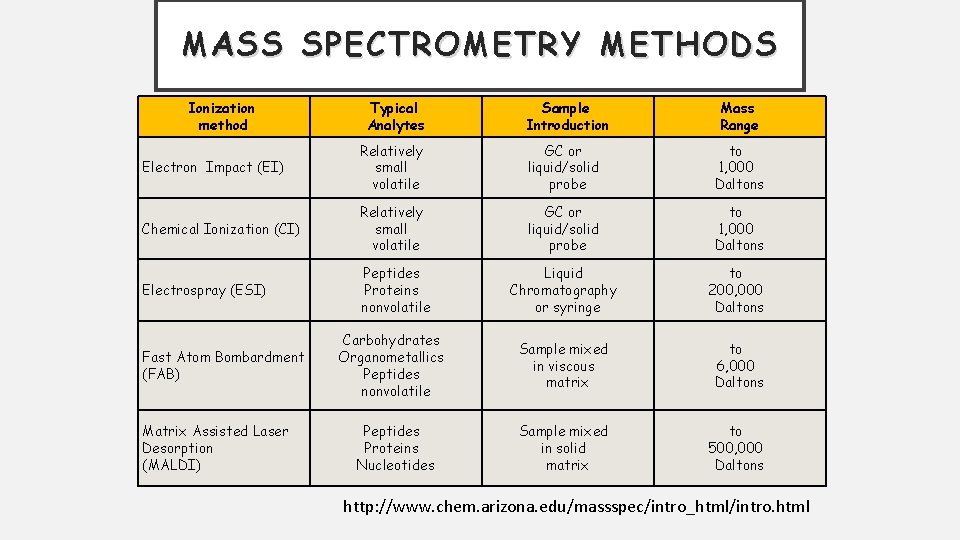
MASS SPECTROMETRY METHODS Ionization method Typical Analytes Sample Introduction Mass Range Electron Impact (EI) Relatively small volatile GC or liquid/solid probe to 1, 000 Daltons Chemical Ionization (CI) Relatively small volatile GC or liquid/solid probe to 1, 000 Daltons Electrospray (ESI) Peptides Proteins nonvolatile Liquid Chromatography or syringe to 200, 000 Daltons Carbohydrates Organometallics Peptides nonvolatile Sample mixed in viscous matrix to 6, 000 Daltons Peptides Proteins Nucleotides Sample mixed in solid matrix to 500, 000 Daltons Fast Atom Bombardment (FAB) Matrix Assisted Laser Desorption (MALDI) http: //www. chem. arizona. edu/massspec/intro_html/intro. html
![MASS SPECTRUM OF CO 2 Molecular ion peak CO 2 44 Fragment Peaks MASS SPECTRUM OF CO 2 Molecular ion peak [CO 2]+ = 44 Fragment Peaks](https://slidetodoc.com/presentation_image_h/2e8d2de15fdb851dae58db67505cb7a1/image-36.jpg)
MASS SPECTRUM OF CO 2 Molecular ion peak [CO 2]+ = 44 Fragment Peaks [C]+ = 12 [O]+ = 16 [CO]+ = 28

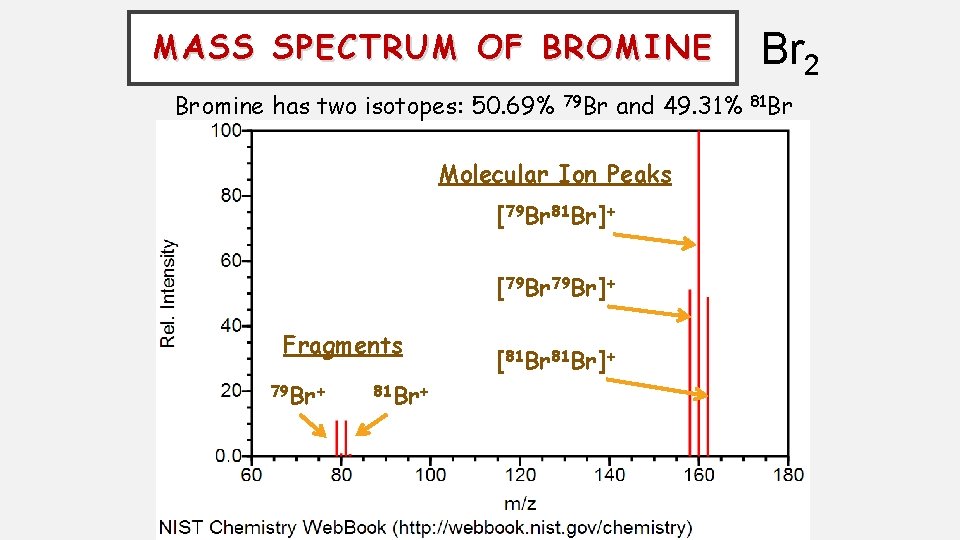
MASS SPECTRUM OF BROMINE Bromine has two isotopes: 50. 69% 79 Br and 49. 31% Molecular Ion Peaks [79 Br 81 Br]+ [79 Br]+ Fragments 79 Br+ 81 Br+ [81 Br]+ Br 2 81 Br

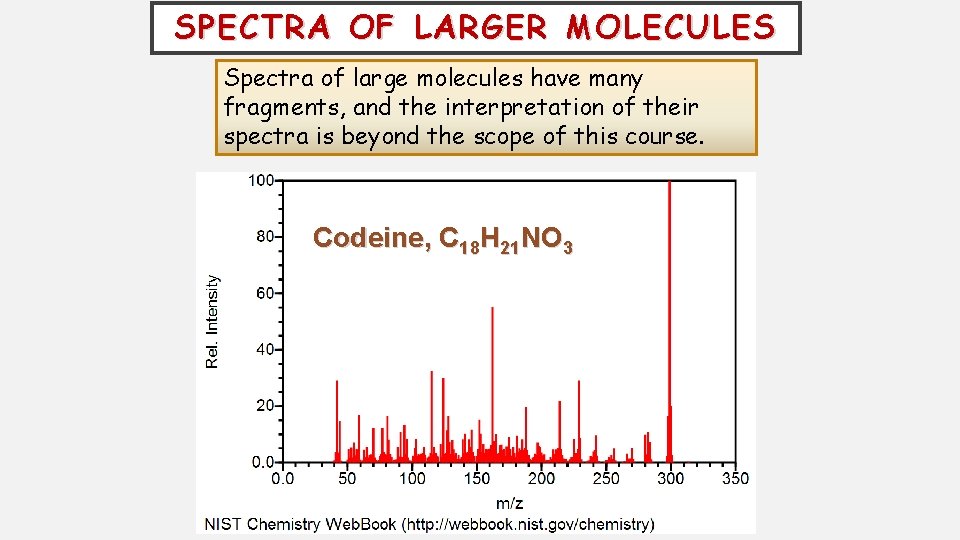
SPECTRA OF LARGER MOLECULES Spectra of large molecules have many fragments, and the interpretation of their spectra is beyond the scope of this course. Codeine, C 18 H 21 NO 3

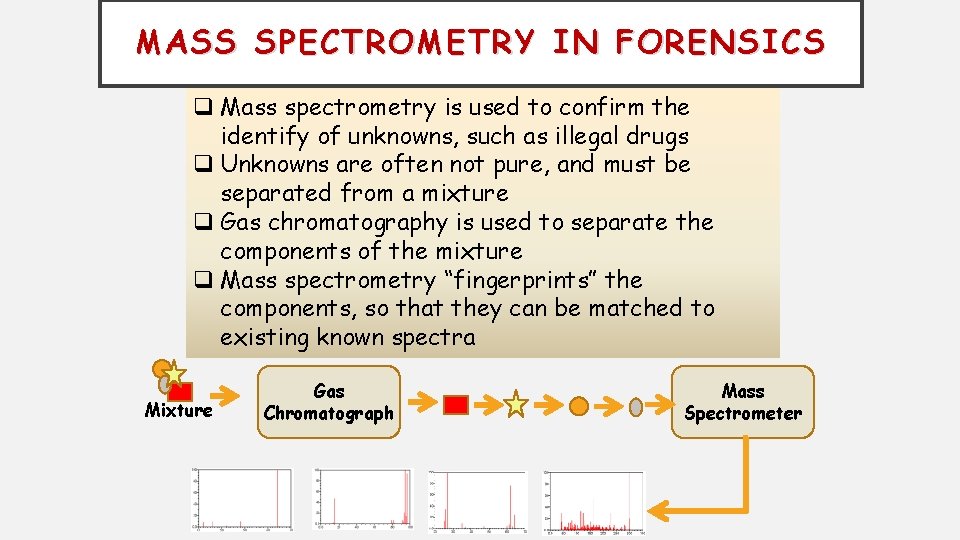
MASS SPECTROMETRY IN FORENSICS q Mass spectrometry is used to confirm the identify of unknowns, such as illegal drugs q Unknowns are often not pure, and must be separated from a mixture q Gas chromatography is used to separate the components of the mixture q Mass spectrometry “fingerprints” the components, so that they can be matched to existing known spectra Mixture Gas Chromatograph Mass Spectrometer

ISOTOPE • Atoms which have the same Z (same # p+) but a different A (different # n 0) • Most elements have isotopes that occur in nature in precise proportions (fractional abundances, %).

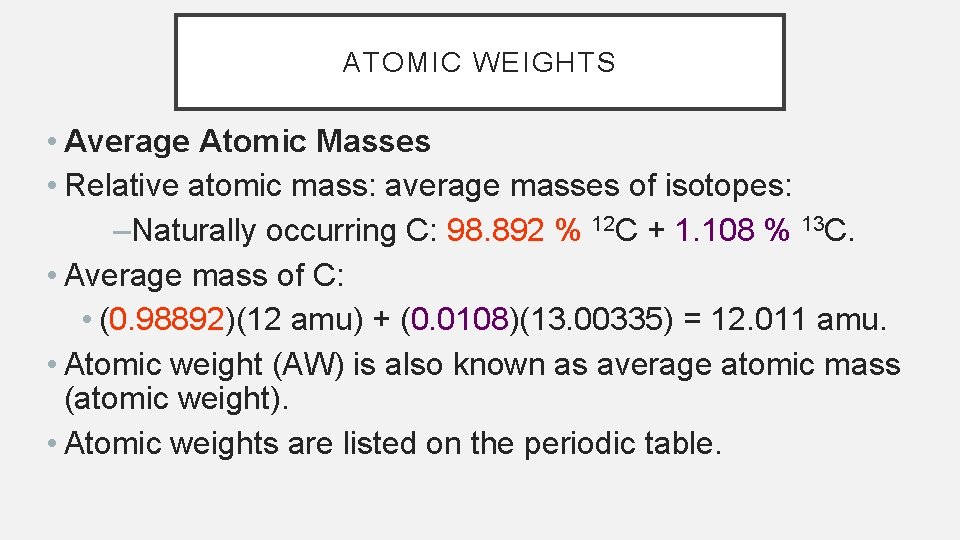
ATOMIC WEIGHTS • Average Atomic Masses • Relative atomic mass: average masses of isotopes: –Naturally occurring C: 98. 892 % 12 C + 1. 108 % 13 C. • Average mass of C: • (0. 98892)(12 amu) + (0. 0108)(13. 00335) = 12. 011 amu. • Atomic weight (AW) is also known as average atomic mass (atomic weight). • Atomic weights are listed on the periodic table.

DO THESE • Questions: 2. 24, 2. 26, 2. 28

1. 3 ELEMENTAL COMPOSITION OF PURE SUBSTANCES BL 3. 5, 1. 2 Questions: 3. 42, 3. 46, 3. 50

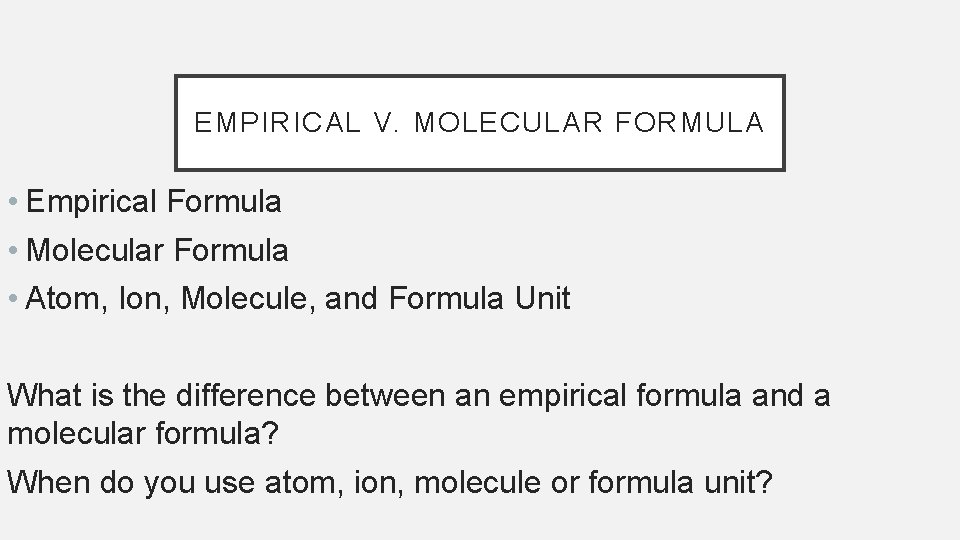
EMPIRICAL V. MOLECULAR FORMULA • Empirical Formula • Molecular Formula • Atom, Ion, Molecule, and Formula Unit What is the difference between an empirical formula and a molecular formula? When do you use atom, ion, molecule or formula unit?

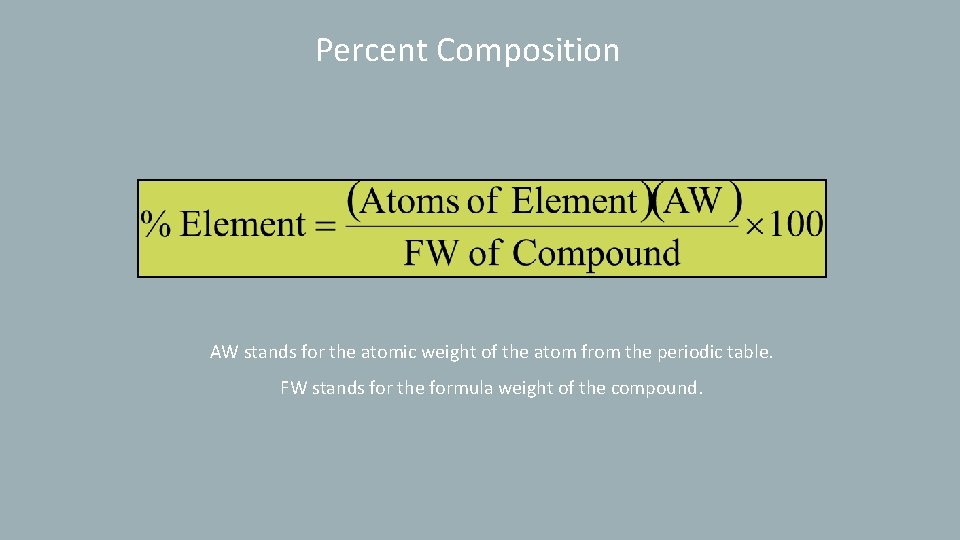
Percent Composition AW stands for the atomic weight of the atom from the periodic table. FW stands for the formula weight of the compound.

FREONS ARE GASEOUS COMPOUNDS USED IN REFRIGERATION. A PARTICULAR FREON CONTAINS 9. 93% CARBON, 58. 6% CHLORINE, AND 31. 4% FLUORINE BY MASS. WHAT CAN BE FOUND WITH THIS INFORMATION?

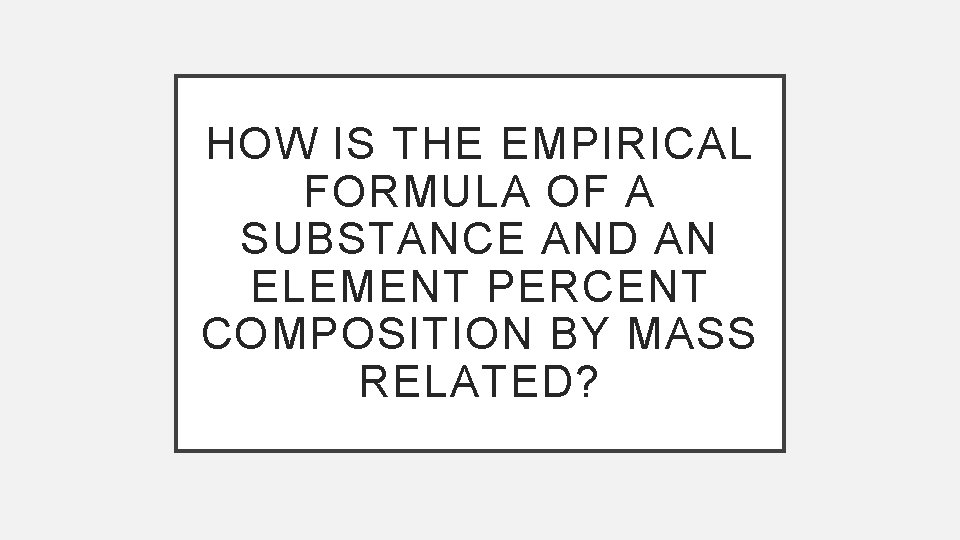
HOW IS THE EMPIRICAL FORMULA OF A SUBSTANCE AND AN ELEMENT PERCENT COMPOSITION BY MASS RELATED?

PARTICLE REPRESENTATION • Molecule; Covalent Compound • Formula Unit; Ionic Compound

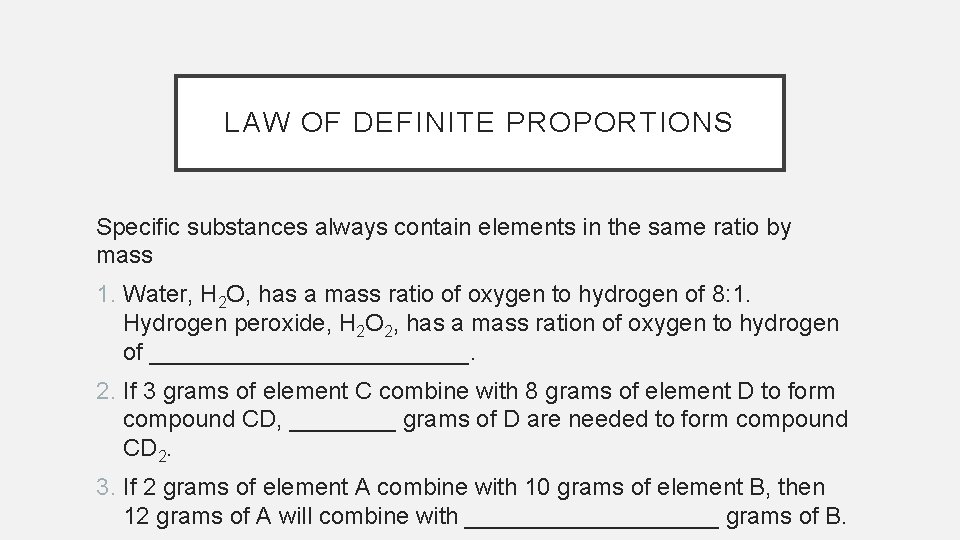
LAW OF DEFINITE PROPORTIONS Specific substances always contain elements in the same ratio by mass 1. Water, H 2 O, has a mass ratio of oxygen to hydrogen of 8: 1. Hydrogen peroxide, H 2 O 2, has a mass ration of oxygen to hydrogen of ____________. 2. If 3 grams of element C combine with 8 grams of element D to form compound CD, ____ grams of D are needed to form compound CD 2. 3. If 2 grams of element A combine with 10 grams of element B, then 12 grams of A will combine with __________ grams of B.

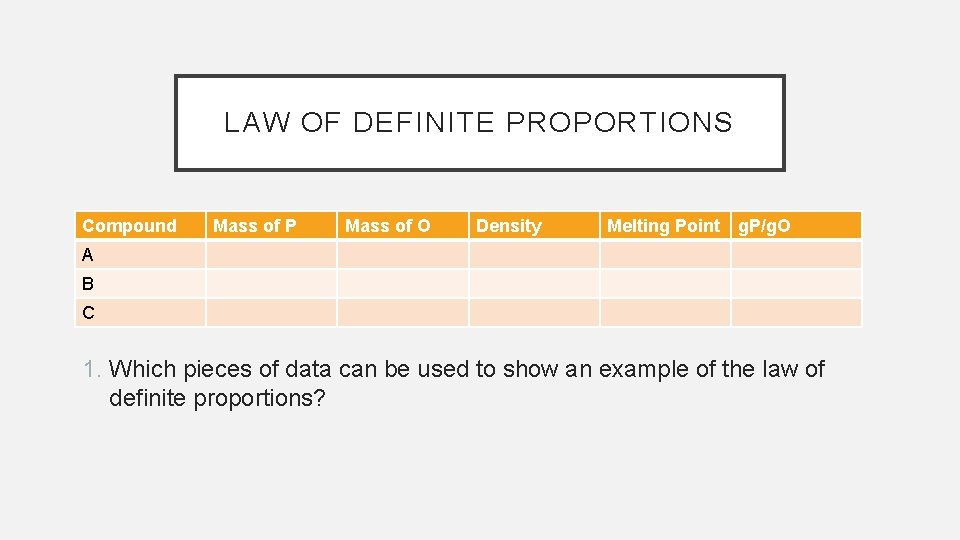
LAW OF DEFINITE PROPORTIONS Compound Mass of P Mass of O Density Melting Point g. P/g. O A B C 1. Which pieces of data can be used to show an example of the law of definite proportions?

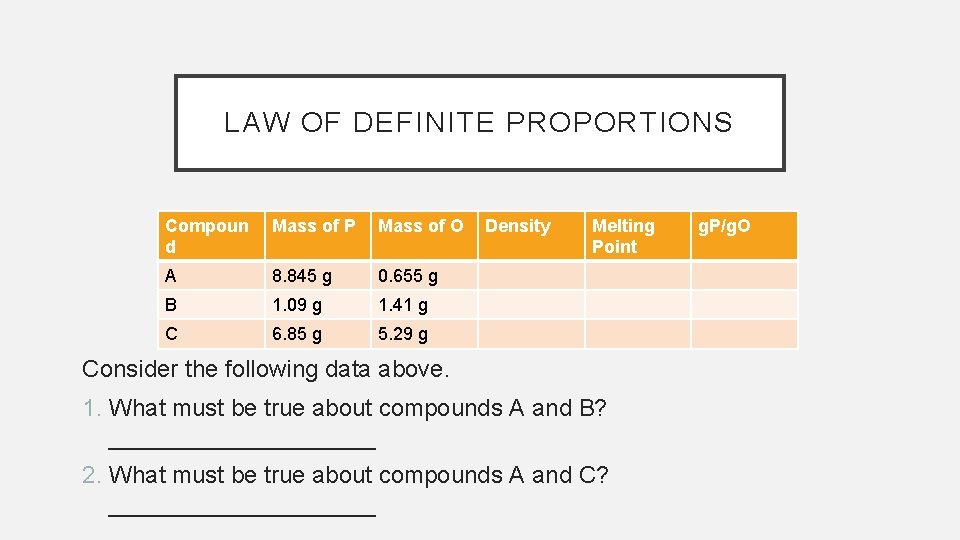
LAW OF DEFINITE PROPORTIONS Compoun d Mass of P Mass of O A 8. 845 g 0. 655 g B 1. 09 g 1. 41 g C 6. 85 g 5. 29 g Density Melting Point Consider the following data above. 1. What must be true about compounds A and B? __________ 2. What must be true about compounds A and C? __________ g. P/g. O

CERR ON ELEMENTAL COMPOSITION • You have made your claim. Get together with your group to give Evidence, Reasoning, and a Rebuttal. Use the terms percent composition, empirical formula, and law of definite proportions somewhere in your work.

1. 4 COMPOSITION OF MIXTURES BL 1. 2,

MIXTURES V SUBSTANCES • Mixtures have a _______ composition while pure substances have a _____ composition.

ELEMENTAL ANALYSIS • By knowing the molar mass of elements the percent composition of each element in a compound can be found. This can further be used to draw conclusions about a sample of a substance that contains impurities. • An impure sample of SO 3 contains 40% Oxygen. What is the percent of SO 3 in the sample?

Chemistry, The Central Science, 11 th edition Theodore L. Brown; H. Eugene Le. May, Jr. ; and Bruce E. Bursten CHAPTER 6 ELECTRONIC STRUCTURE OF ATOMS John D. Bookstaver St. Charles Community College Cottleville, MO © 2009, Prentice-Hall, Inc.

1. 5 ATOMIC STRUCTURE AND ELECTRON CONFIGURATION Chapter 6 Questions: 6. 6, 6. 10, 6. 14, 6. 18, 6. 22, 6. 28, 6. 36, 6. 42, 6. 46, 6. 56, 6. 62, 6. 70, 6. 74, 6. 78, 6. 90

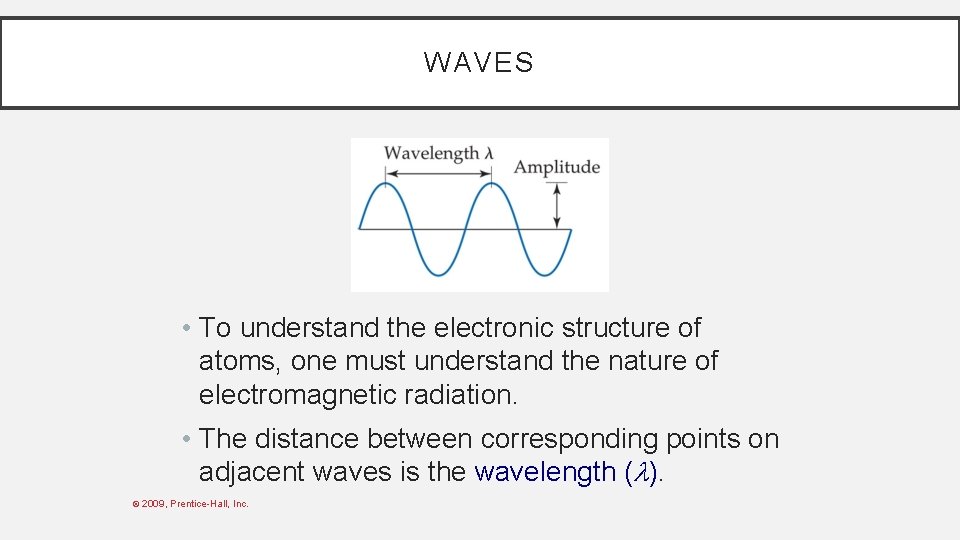
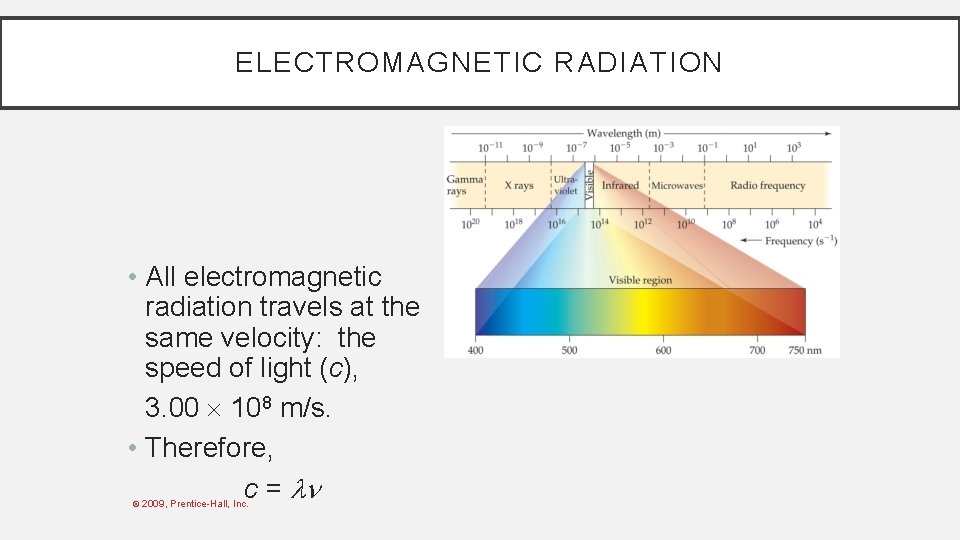
WAVES • To understand the electronic structure of atoms, one must understand the nature of electromagnetic radiation. • The distance between corresponding points on adjacent waves is the wavelength ( ). © 2009, Prentice-Hall, Inc.

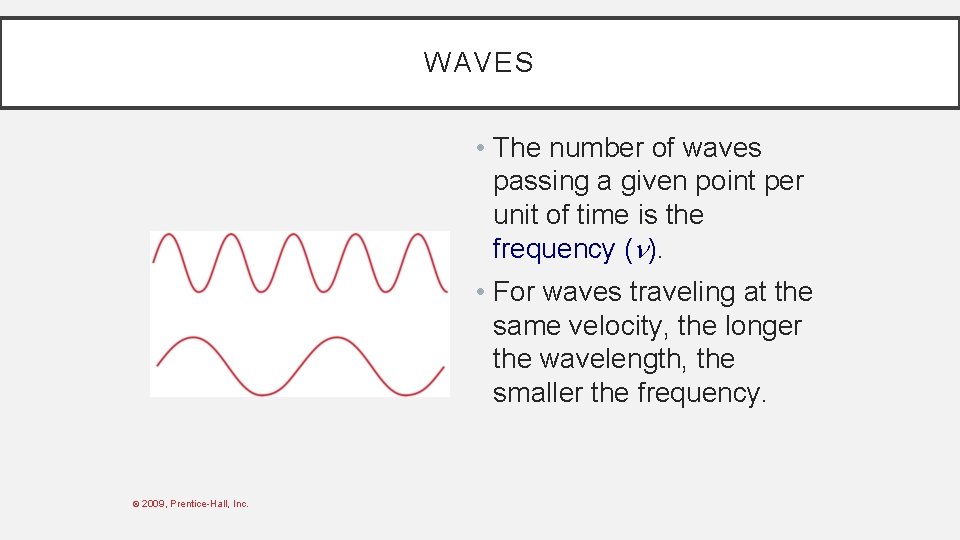
WAVES • The number of waves passing a given point per unit of time is the frequency ( ). • For waves traveling at the same velocity, the longer the wavelength, the smaller the frequency. © 2009, Prentice-Hall, Inc.

ELECTROMAGNETIC RADIATION • All electromagnetic radiation travels at the same velocity: the speed of light (c), 3. 00 108 m/s. • Therefore, c = © 2009, Prentice-Hall, Inc.

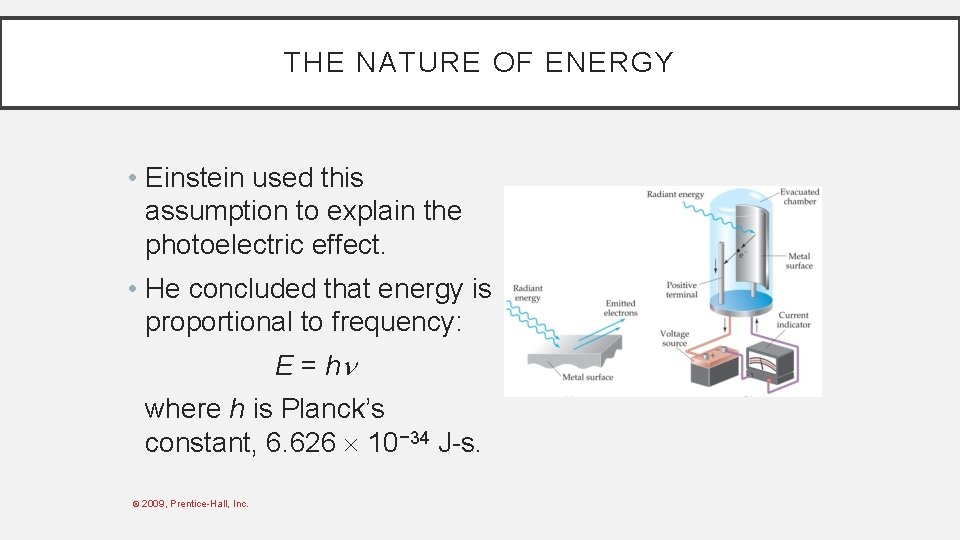
THE NATURE OF ENERGY • The wave nature of light does not explain how an object can glow when its temperature increases. • Max Planck explained it by assuming that energy comes in packets called quanta. © 2009, Prentice-Hall, Inc.

THE NATURE OF ENERGY • Einstein used this assumption to explain the photoelectric effect. • He concluded that energy is proportional to frequency: E = h where h is Planck’s constant, 6. 626 10− 34 J-s. © 2009, Prentice-Hall, Inc.

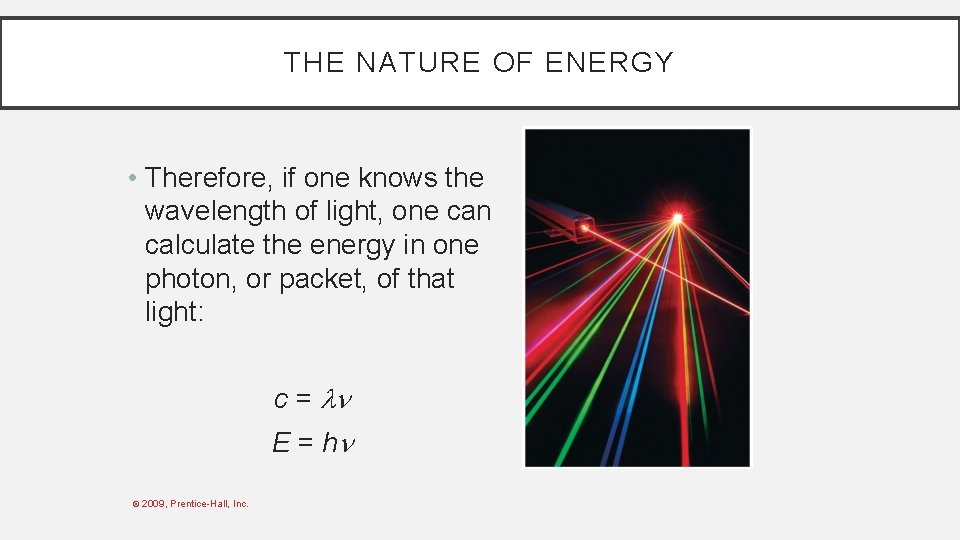
THE NATURE OF ENERGY • Therefore, if one knows the wavelength of light, one can calculate the energy in one photon, or packet, of that light: c = E = h © 2009, Prentice-Hall, Inc.

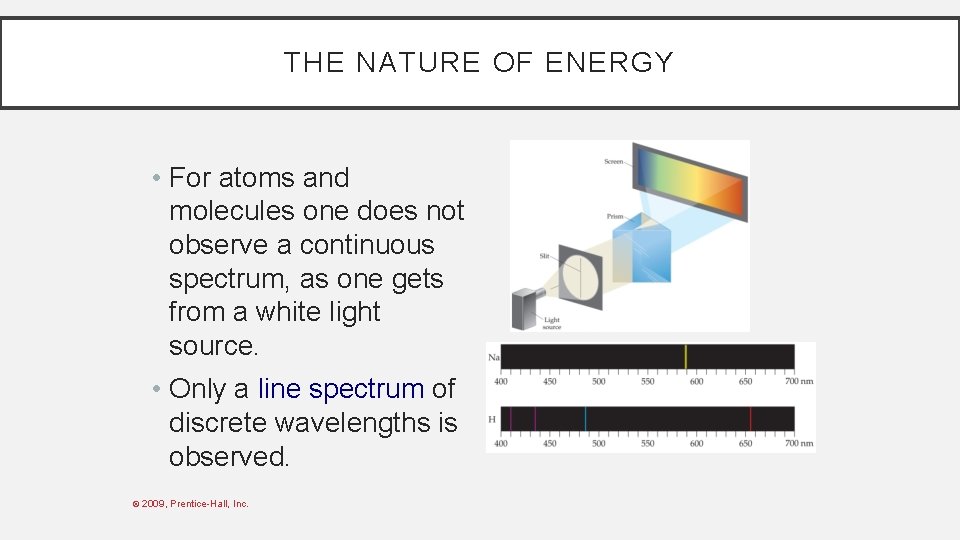
THE NATURE OF ENERGY Another mystery in the early 20 th century involved the emission spectra observed from energy emitted by atoms and molecules. © 2009, Prentice-Hall, Inc.

THE NATURE OF ENERGY • For atoms and molecules one does not observe a continuous spectrum, as one gets from a white light source. • Only a line spectrum of discrete wavelengths is observed. © 2009, Prentice-Hall, Inc.

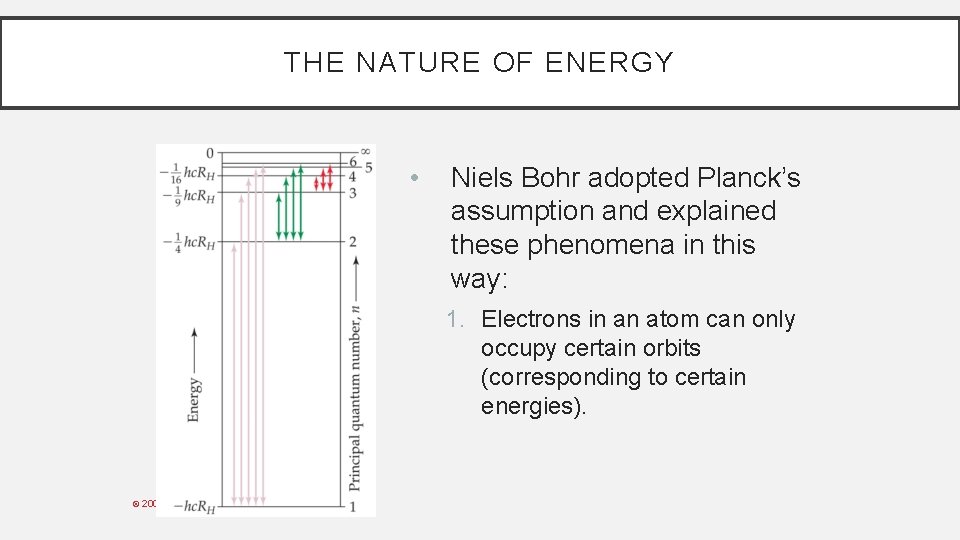
THE NATURE OF ENERGY • Niels Bohr adopted Planck’s assumption and explained these phenomena in this way: 1. Electrons in an atom can only occupy certain orbits (corresponding to certain energies). © 2009, Prentice-Hall, Inc.

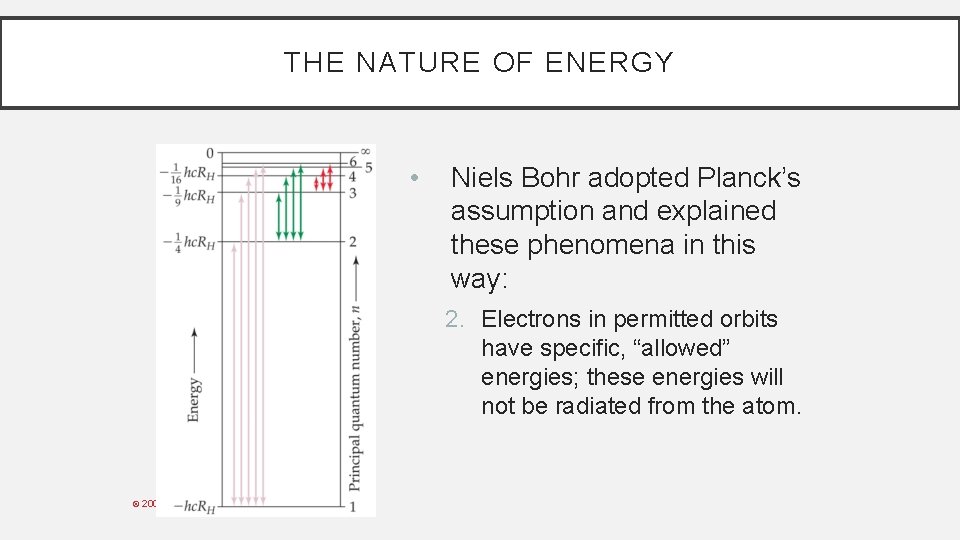
THE NATURE OF ENERGY • Niels Bohr adopted Planck’s assumption and explained these phenomena in this way: 2. Electrons in permitted orbits have specific, “allowed” energies; these energies will not be radiated from the atom. © 2009, Prentice-Hall, Inc.

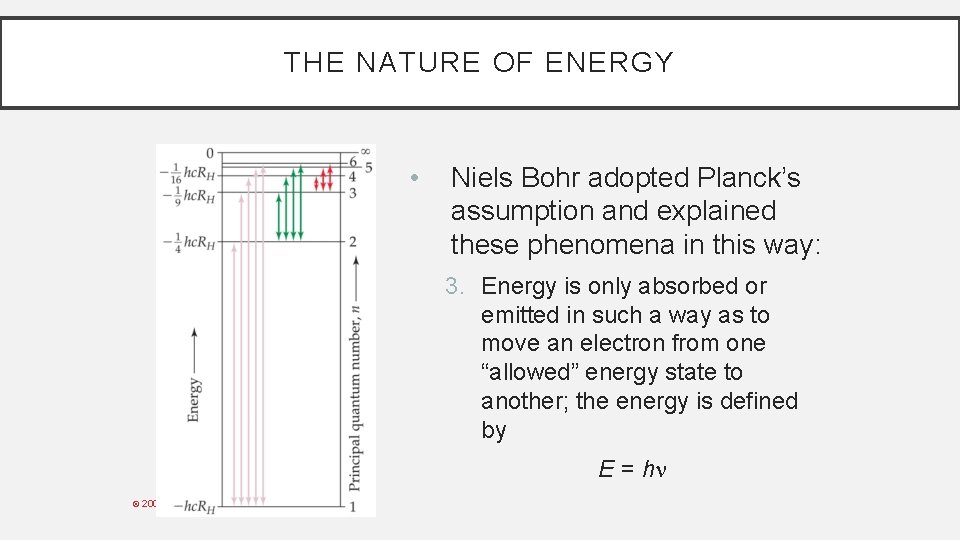
THE NATURE OF ENERGY • Niels Bohr adopted Planck’s assumption and explained these phenomena in this way: 3. Energy is only absorbed or emitted in such a way as to move an electron from one “allowed” energy state to another; the energy is defined by E = h © 2009, Prentice-Hall, Inc.

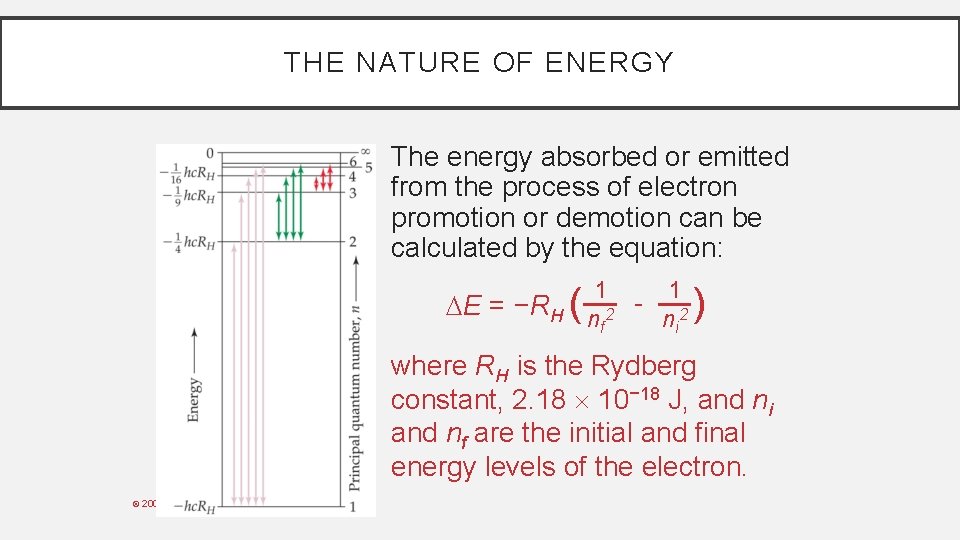
THE NATURE OF ENERGY The energy absorbed or emitted from the process of electron promotion or demotion can be calculated by the equation: 1 1 E = −RH n 2 - n 2 i f ( ) where RH is the Rydberg constant, 2. 18 10− 18 J, and ni and nf are the initial and final energy levels of the electron. © 2009, Prentice-Hall, Inc.

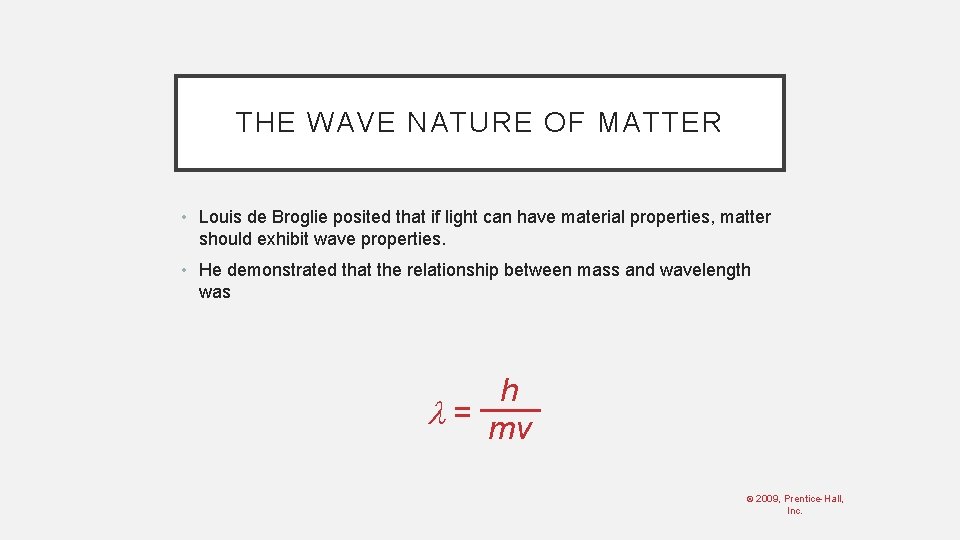
THE WAVE NATURE OF MATTER • Louis de Broglie posited that if light can have material properties, matter should exhibit wave properties. • He demonstrated that the relationship between mass and wavelength was h = mv © 2009, Prentice-Hall, Inc.

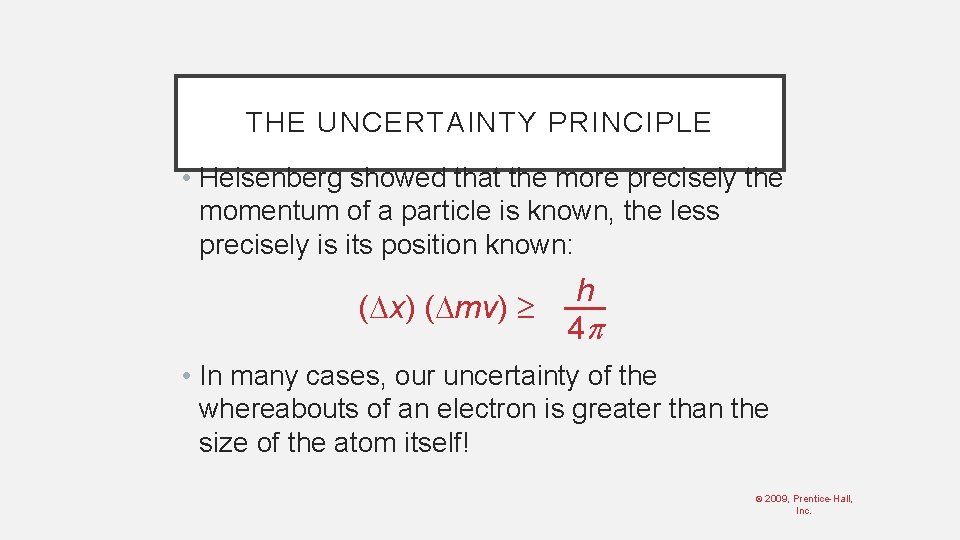
THE UNCERTAINTY PRINCIPLE • Heisenberg showed that the more precisely the momentum of a particle is known, the less precisely is its position known: ( x) ( mv) h 4 • In many cases, our uncertainty of the whereabouts of an electron is greater than the size of the atom itself! © 2009, Prentice-Hall, Inc.

QUANTUM MECHANICS • Erwin Schrödinger developed a mathematical treatment into which both the wave and particle nature of matter could be incorporated. • It is known as quantum mechanics. © 2009, Prentice-Hall, Inc.

QUANTUM MECHANICS • The wave equation is designated with a lower case Greek psi ( ). • The square of the wave equation, 2, gives a probability density map of where an electron has a certain statistical likelihood of being at any given instant in time. © 2009, Prentice-Hall, Inc.

QUANTUM NUMBERS • Solving the wave equation gives a set of wave functions, or orbitals, and their corresponding energies. • Each orbital describes a spatial distribution of electron density. • An orbital is described by a set of three quantum numbers. © 2009, Prentice-Hall, Inc.

PRINCIPAL QUANTUM NUMBER (N) • The principal quantum number, n, describes the energy level on which the orbital resides. • The values of n are integers ≥ 1. © 2009, Prentice-Hall, Inc.

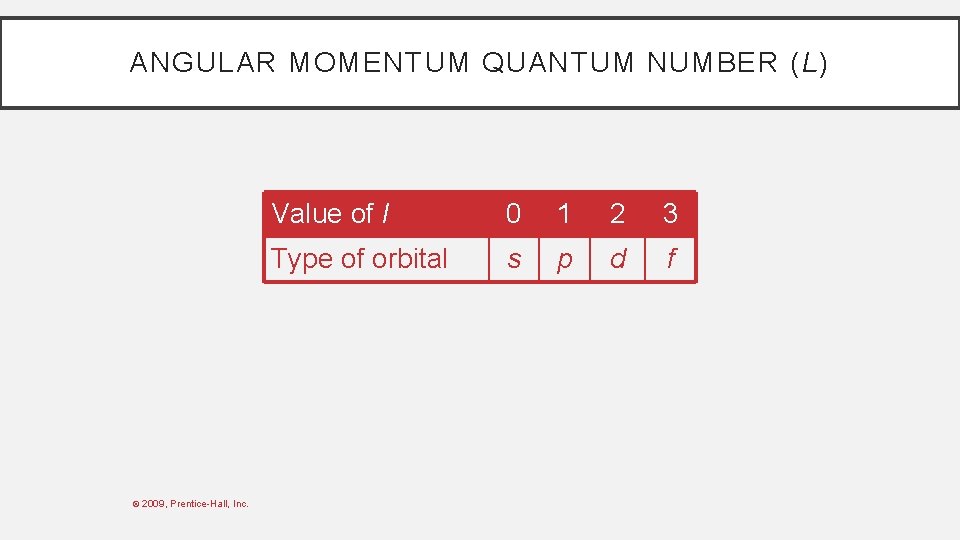
ANGULAR MOMENTUM QUANTUM NUMBER (L) • This quantum number defines the shape of the orbital. • Allowed values of l are integers ranging from 0 to n − 1. • We use letter designations to communicate the different values of l and, therefore, the shapes and types of orbitals. © 2009, Prentice-Hall, Inc.

ANGULAR MOMENTUM QUANTUM NUMBER (L) © 2009, Prentice-Hall, Inc. Value of l 0 1 2 3 Type of orbital s p d f

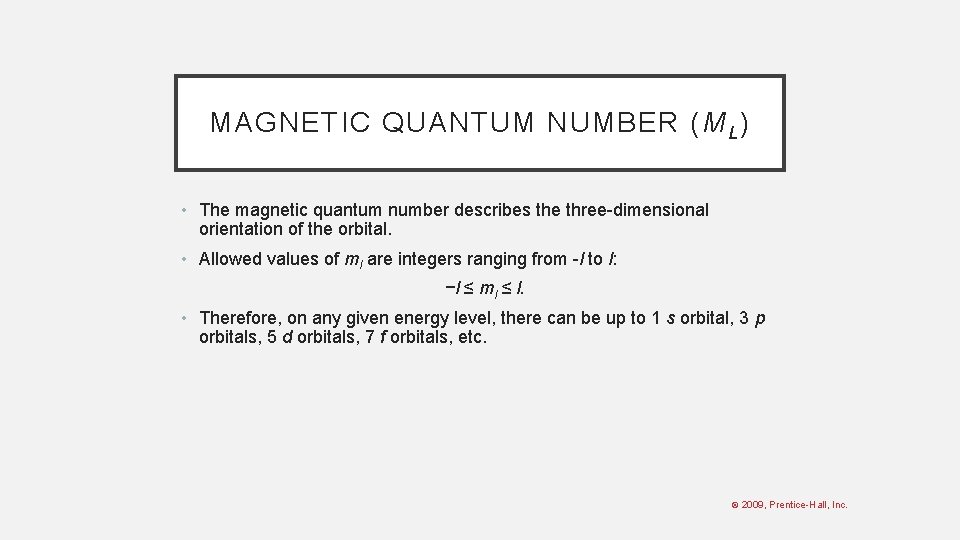
MAGNETIC QUANTUM NUMBER (M L ) • The magnetic quantum number describes the three-dimensional orientation of the orbital. • Allowed values of ml are integers ranging from -l to l: −l ≤ ml ≤ l. • Therefore, on any given energy level, there can be up to 1 s orbital, 3 p orbitals, 5 d orbitals, 7 f orbitals, etc. © 2009, Prentice-Hall, Inc.

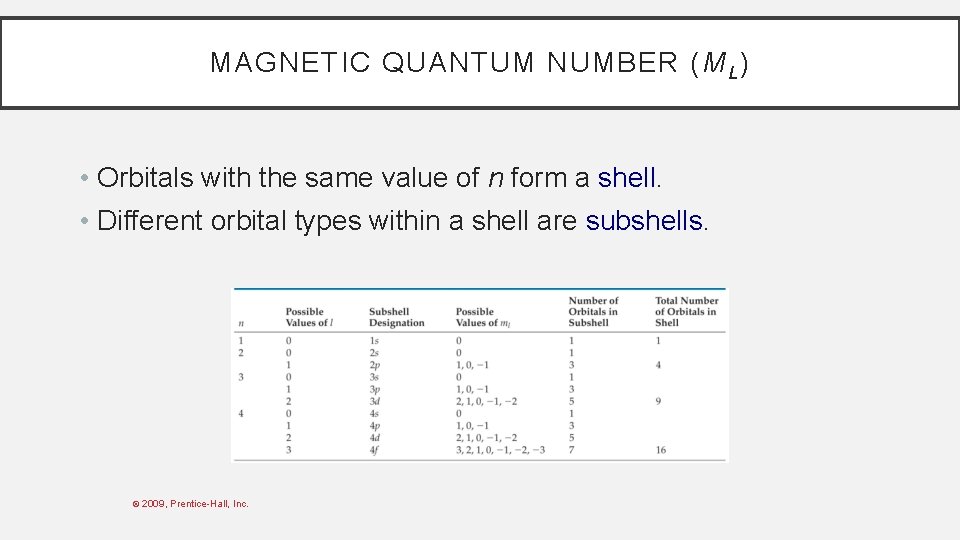
MAGNETIC QUANTUM NUMBER (M L ) • Orbitals with the same value of n form a shell. • Different orbital types within a shell are subshells. © 2009, Prentice-Hall, Inc.

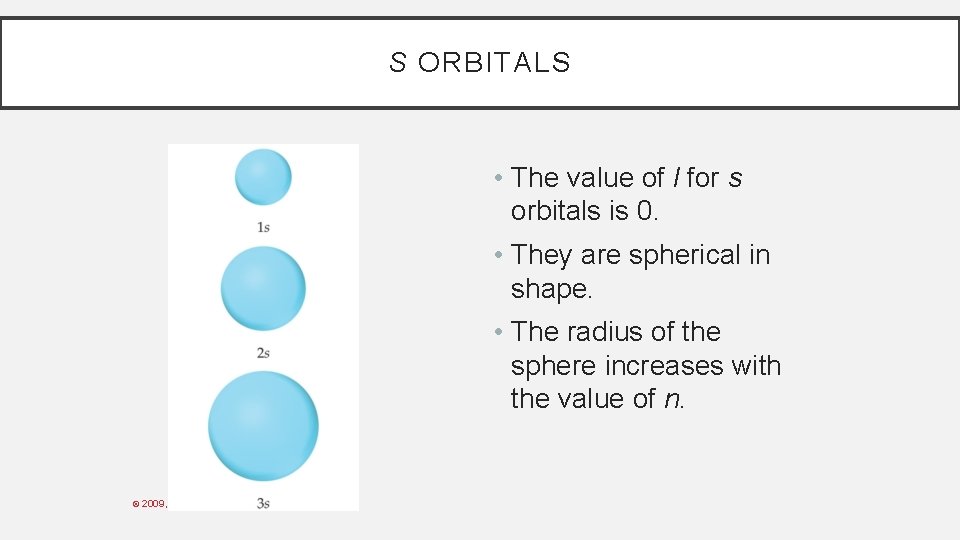
S ORBITALS • The value of l for s orbitals is 0. • They are spherical in shape. • The radius of the sphere increases with the value of n. © 2009, Prentice-Hall, Inc.

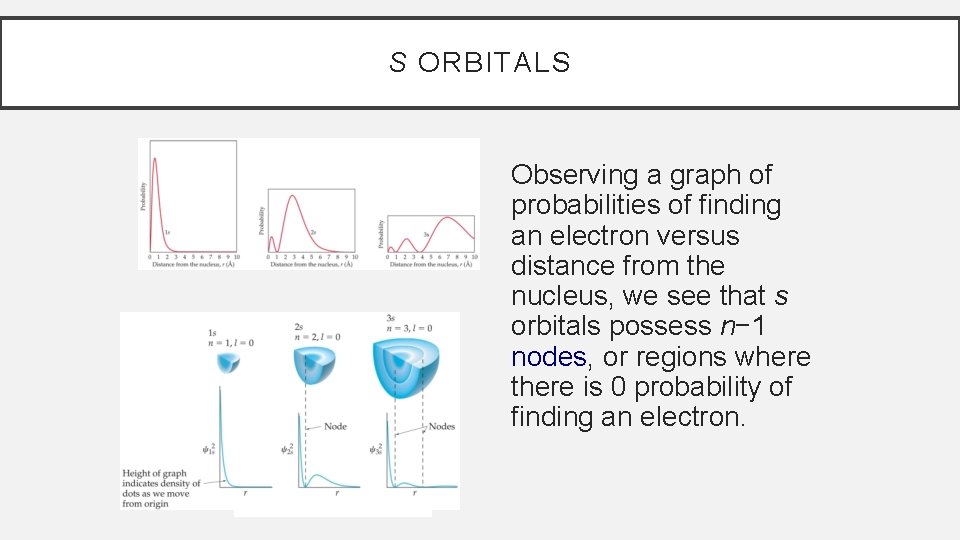
S ORBITALS Observing a graph of probabilities of finding an electron versus distance from the nucleus, we see that s orbitals possess n− 1 nodes, or regions where there is 0 probability of finding an electron. © 2009, Prentice-Hall, Inc.

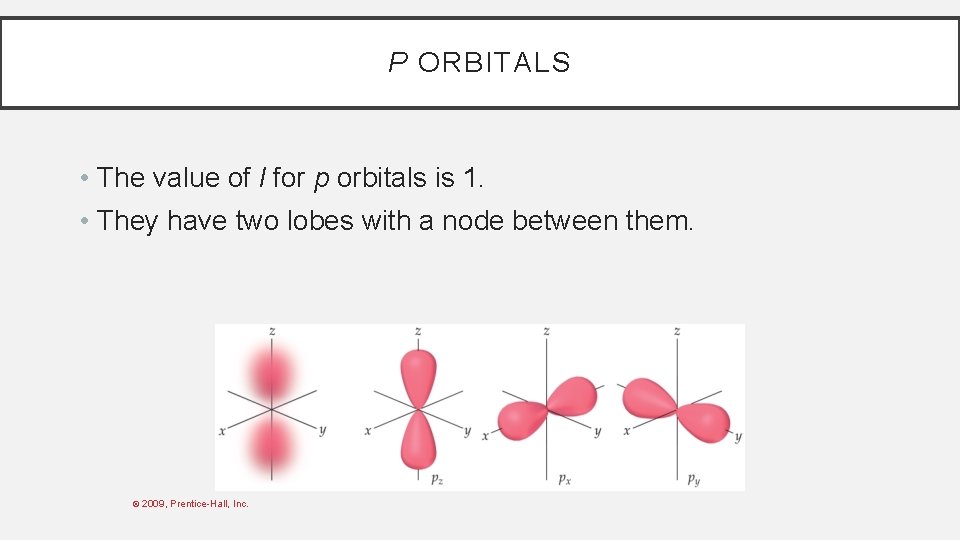
P ORBITALS • The value of l for p orbitals is 1. • They have two lobes with a node between them. © 2009, Prentice-Hall, Inc.

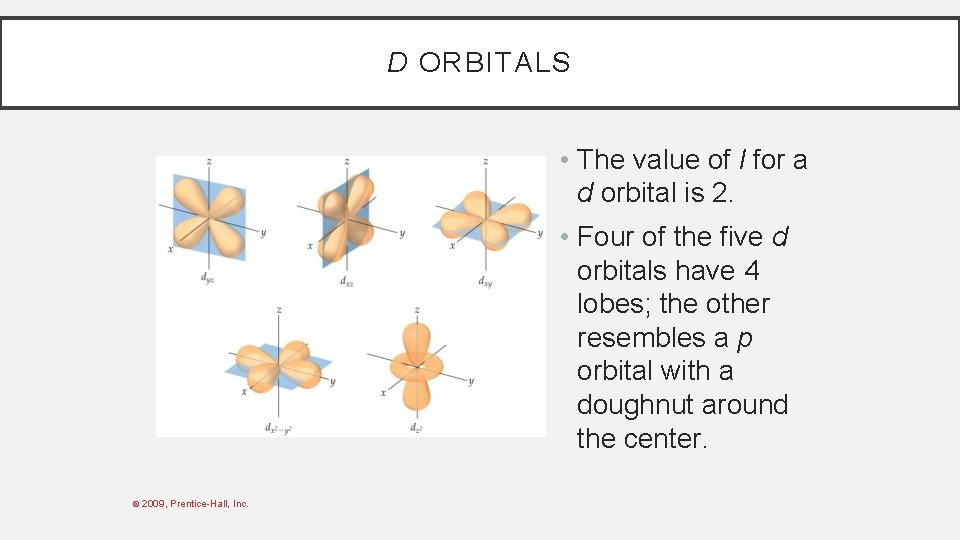
D ORBITALS • The value of l for a d orbital is 2. • Four of the five d orbitals have 4 lobes; the other resembles a p orbital with a doughnut around the center. © 2009, Prentice-Hall, Inc.

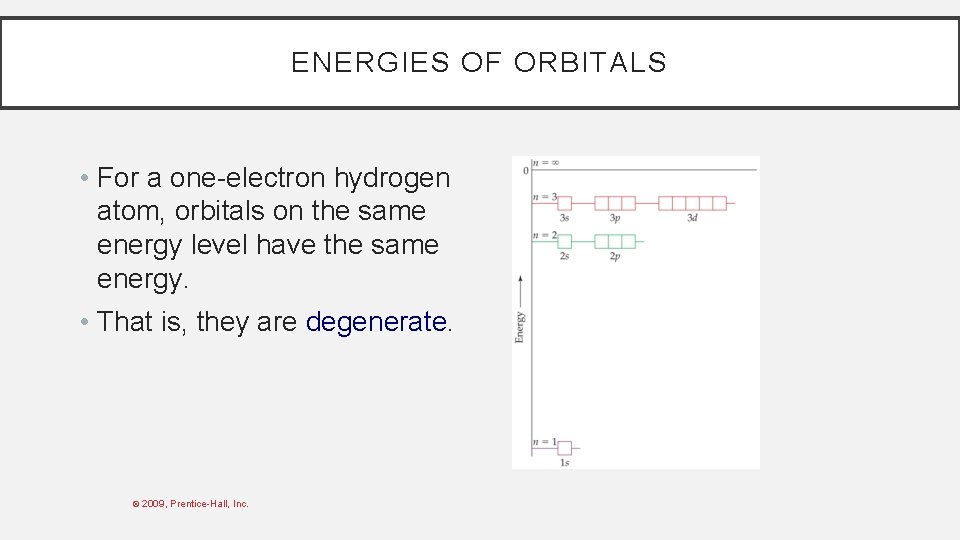
ENERGIES OF ORBITALS • For a one-electron hydrogen atom, orbitals on the same energy level have the same energy. • That is, they are degenerate. © 2009, Prentice-Hall, Inc.

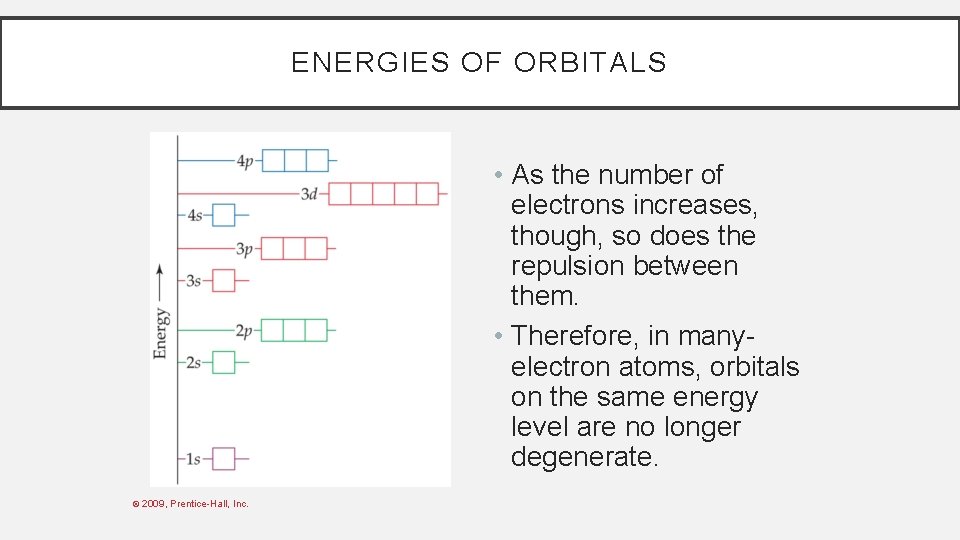
ENERGIES OF ORBITALS • As the number of electrons increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level are no longer degenerate. © 2009, Prentice-Hall, Inc.

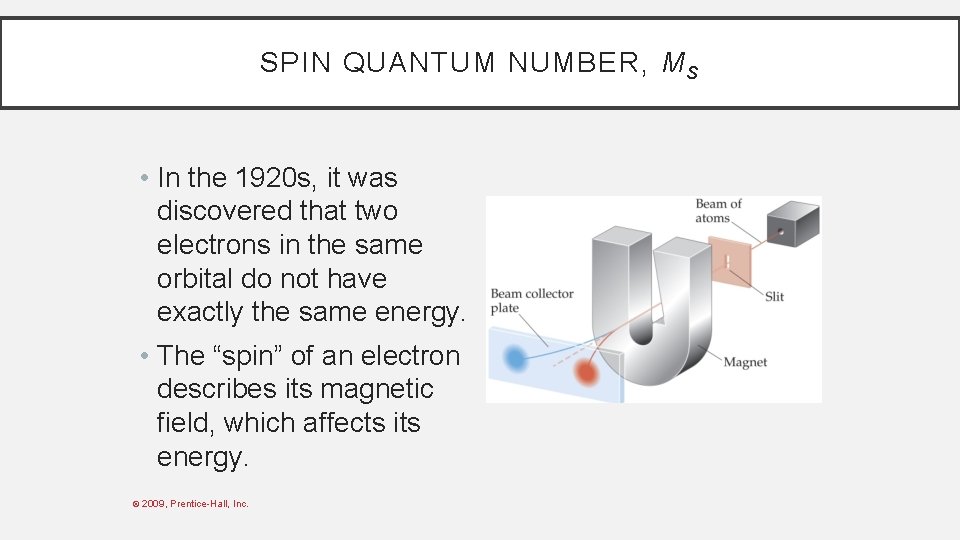
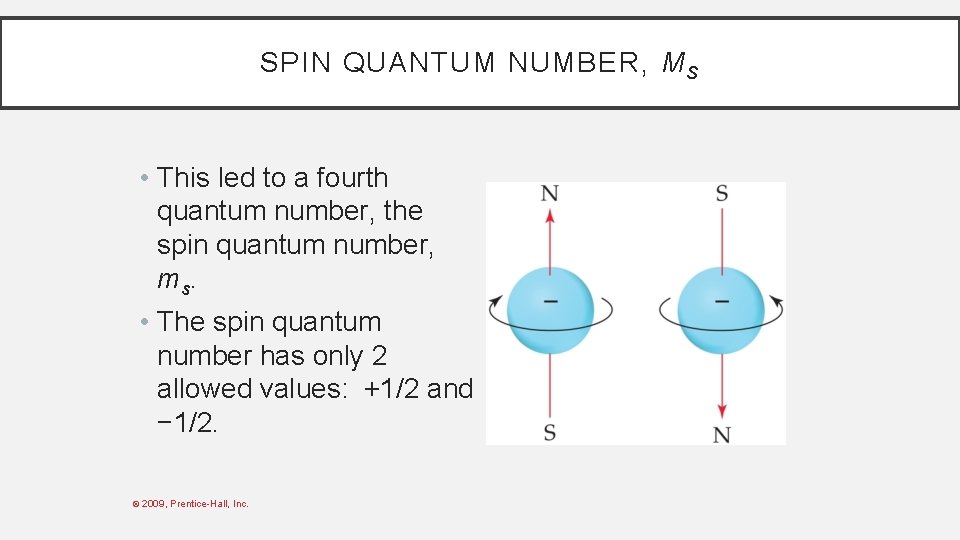
SPIN QUANTUM NUMBER, M S • In the 1920 s, it was discovered that two electrons in the same orbital do not have exactly the same energy. • The “spin” of an electron describes its magnetic field, which affects its energy. © 2009, Prentice-Hall, Inc.

SPIN QUANTUM NUMBER, M S • This led to a fourth quantum number, the spin quantum number, ms. • The spin quantum number has only 2 allowed values: +1/2 and − 1/2. © 2009, Prentice-Hall, Inc.

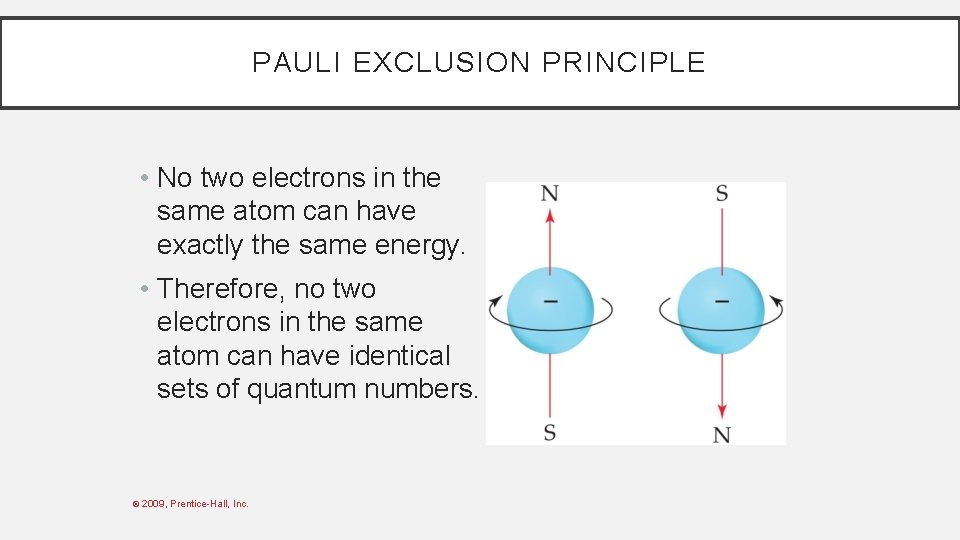
PAULI EXCLUSION PRINCIPLE • No two electrons in the same atom can have exactly the same energy. • Therefore, no two electrons in the same atom can have identical sets of quantum numbers. © 2009, Prentice-Hall, Inc.

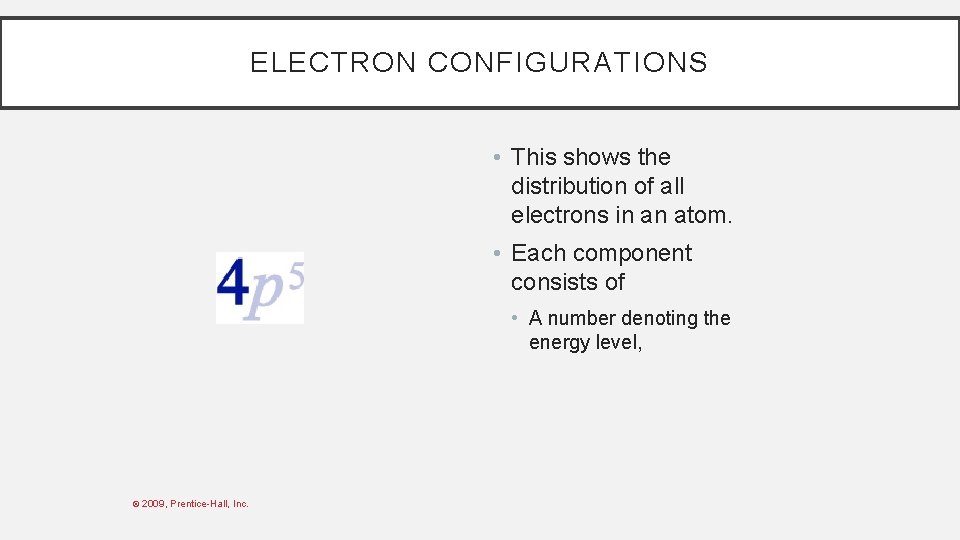
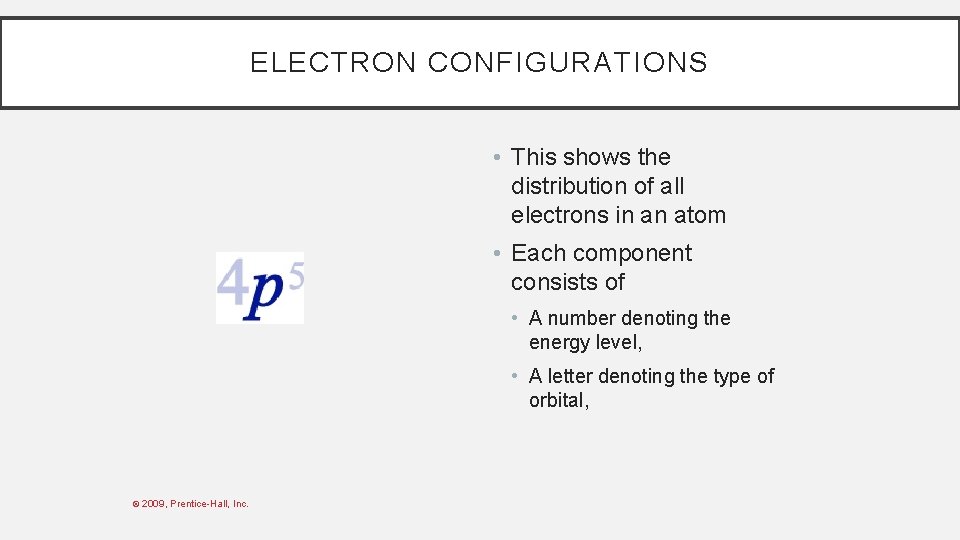
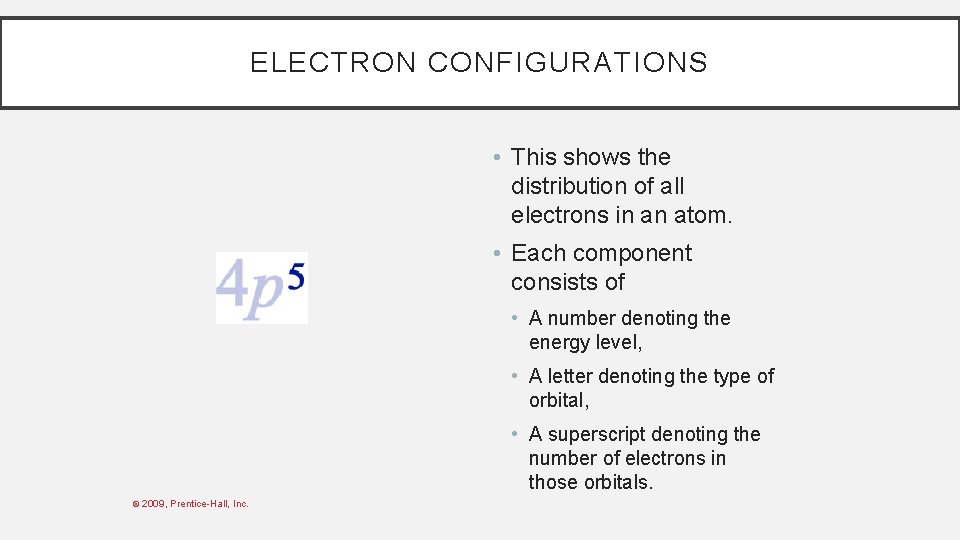
ELECTRON CONFIGURATIONS • This shows the distribution of all electrons in an atom. • Each component consists of • A number denoting the energy level, © 2009, Prentice-Hall, Inc.

ELECTRON CONFIGURATIONS • This shows the distribution of all electrons in an atom • Each component consists of • A number denoting the energy level, • A letter denoting the type of orbital, © 2009, Prentice-Hall, Inc.

ELECTRON CONFIGURATIONS • This shows the distribution of all electrons in an atom. • Each component consists of • A number denoting the energy level, • A letter denoting the type of orbital, • A superscript denoting the number of electrons in those orbitals. © 2009, Prentice-Hall, Inc.

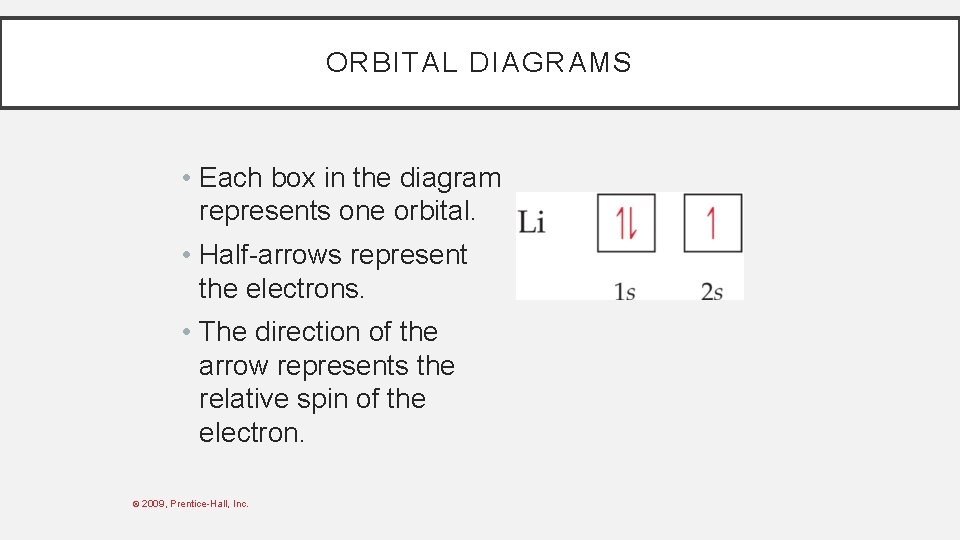
ORBITAL DIAGRAMS • Each box in the diagram represents one orbital. • Half-arrows represent the electrons. • The direction of the arrow represents the relative spin of the electron. © 2009, Prentice-Hall, Inc.

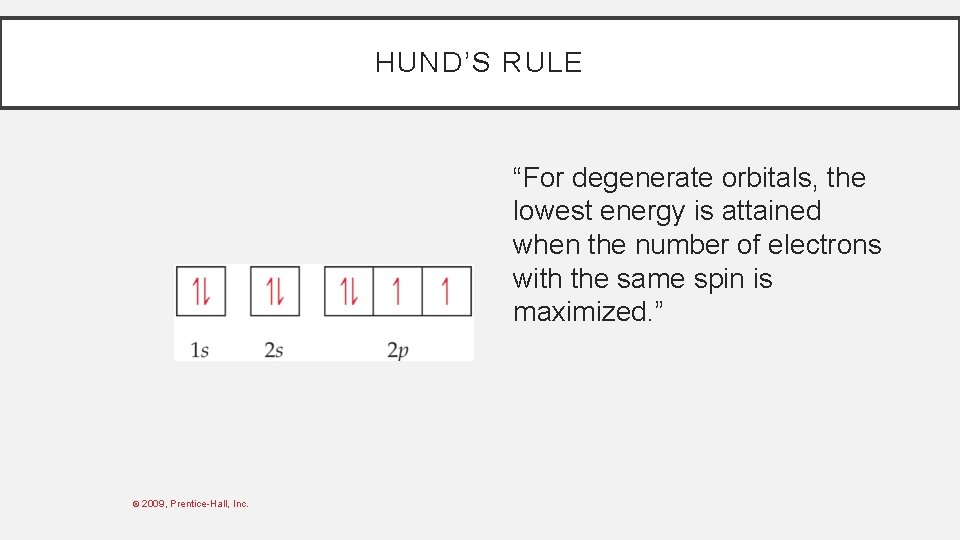
HUND’S RULE “For degenerate orbitals, the lowest energy is attained when the number of electrons with the same spin is maximized. ” © 2009, Prentice-Hall, Inc.

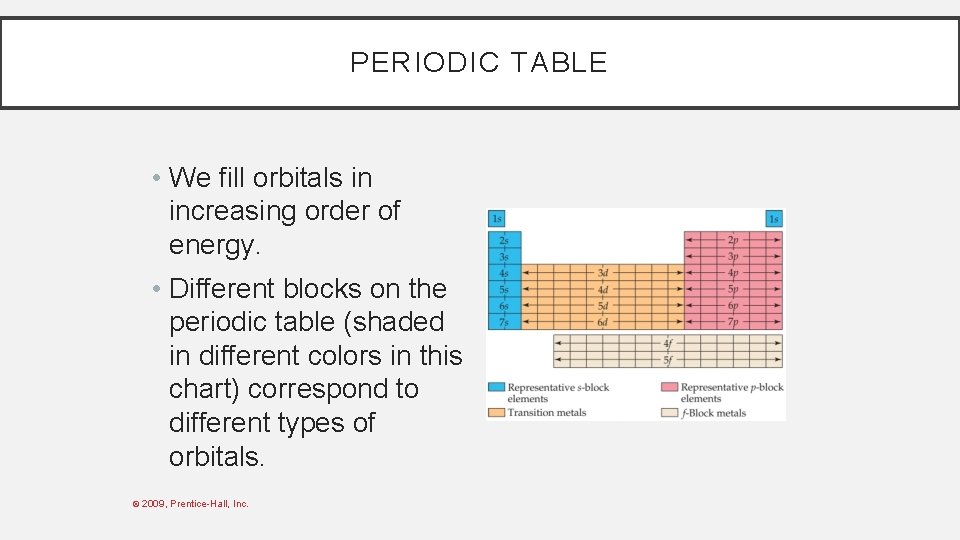
PERIODIC TABLE • We fill orbitals in increasing order of energy. • Different blocks on the periodic table (shaded in different colors in this chart) correspond to different types of orbitals. © 2009, Prentice-Hall, Inc.

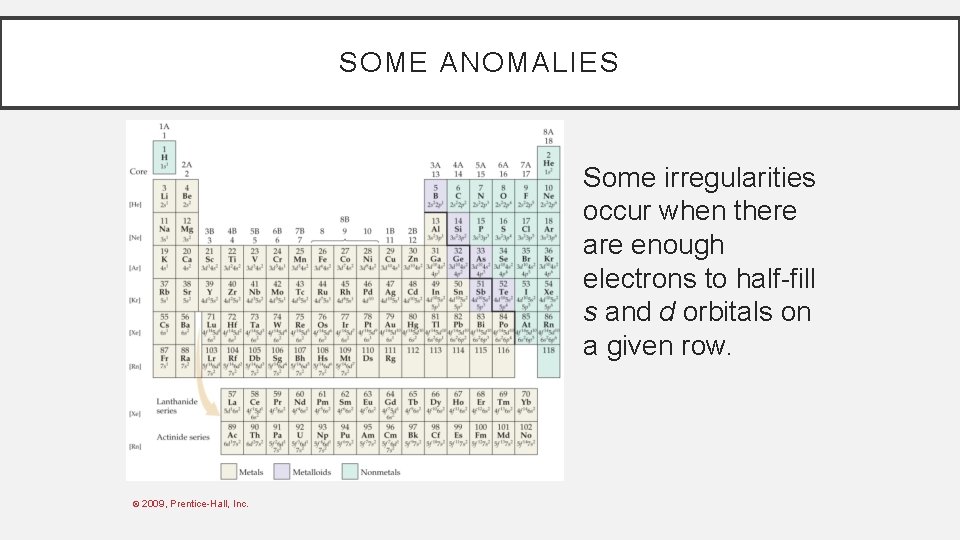
SOME ANOMALIES Some irregularities occur when there are enough electrons to half-fill s and d orbitals on a given row. © 2009, Prentice-Hall, Inc.
![SOME ANOMALIES For instance the electron configuration for copper is Ar 4 s 1 SOME ANOMALIES For instance, the electron configuration for copper is [Ar] 4 s 1](https://slidetodoc.com/presentation_image_h/2e8d2de15fdb851dae58db67505cb7a1/image-96.jpg)
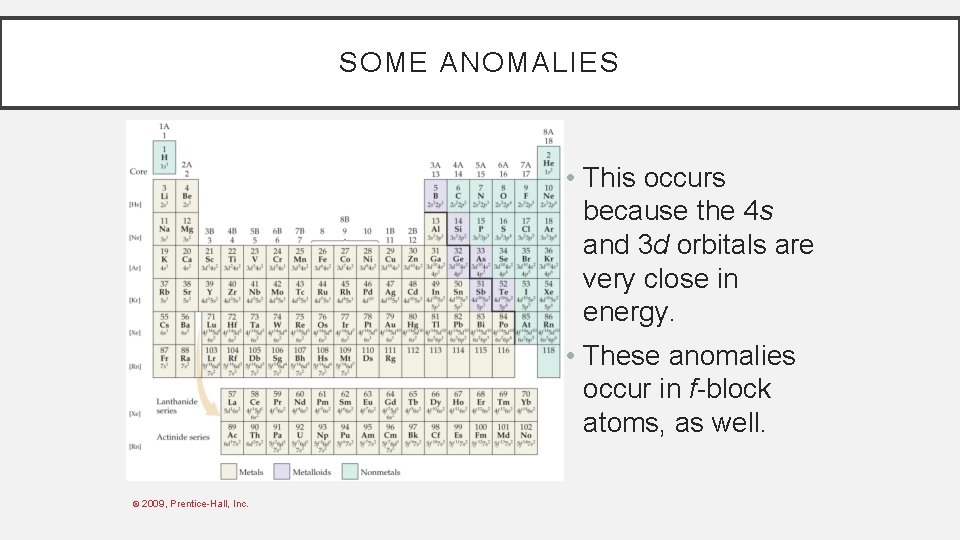
SOME ANOMALIES For instance, the electron configuration for copper is [Ar] 4 s 1 3 d 5 rather than the expected [Ar] 4 s 2 3 d 4. © 2009, Prentice-Hall, Inc.

SOME ANOMALIES • This occurs because the 4 s and 3 d orbitals are very close in energy. • These anomalies occur in f-block atoms, as well. © 2009, Prentice-Hall, Inc.

1. 6 PHOTOELECTRON SPECTROSCOPY

1. 7 PERIODIC TRENDS

1. 8 VALENCE ELECTRONS AND IONIC COMPOUNDS