The History of Atomic Theory Democritus 460 370








- Slides: 21

The History of Atomic Theory

Democritus 460 -370 B. C. • Believed the universe was made of empty space and tiny bits of stuff called atoms that couldn’t be divided into smaller pieces. • The term atom comes from, atomos, which means, “cannot be divided”.

John Dalton-1808 • Believed that matter was made of atoms of different elements. • Considered the father of modern atomic theory.

Thomson's Model Think or positive, negative • In 1897, J. J Thomson passed an electric current through a gas. • He found that the gas gave off rays made up of negatively charged particles. • Prior to this, atomic theory stated that atoms are neutral, there must also be positively charged particles inside an atom.

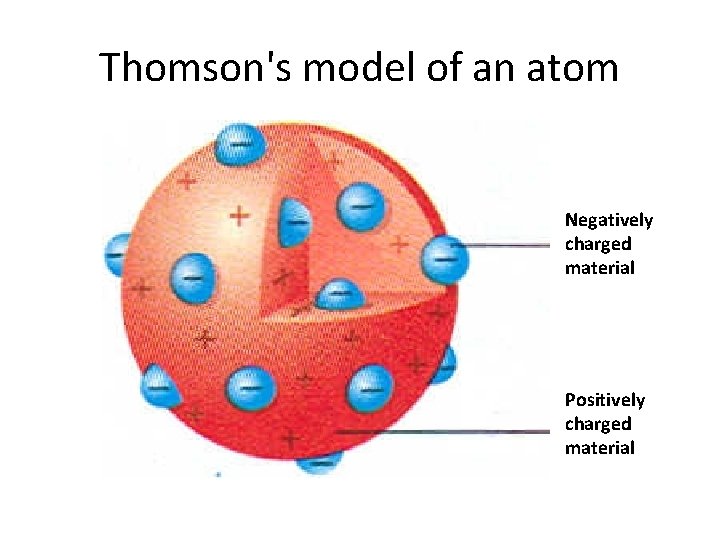
Thomson-1897 • Thomson hypothesized that an atom was made up of a positively charged material with negatively charged particles scattered evenly throughout.

Thomson's model of an atom Negatively charged material Positively charged material

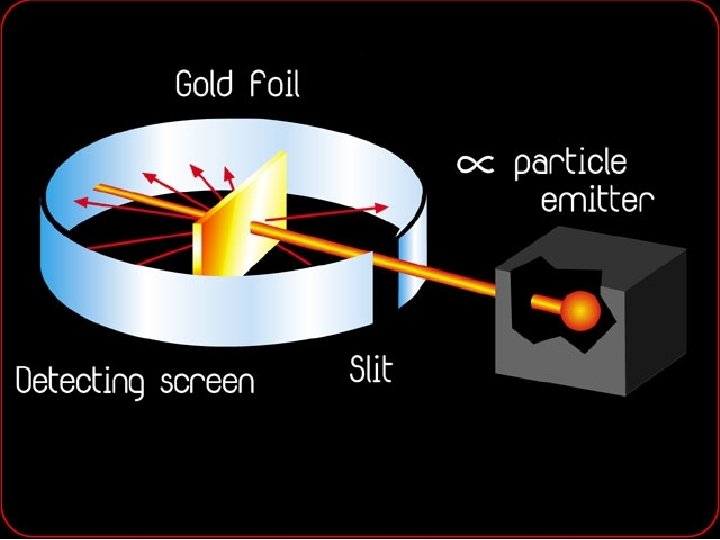
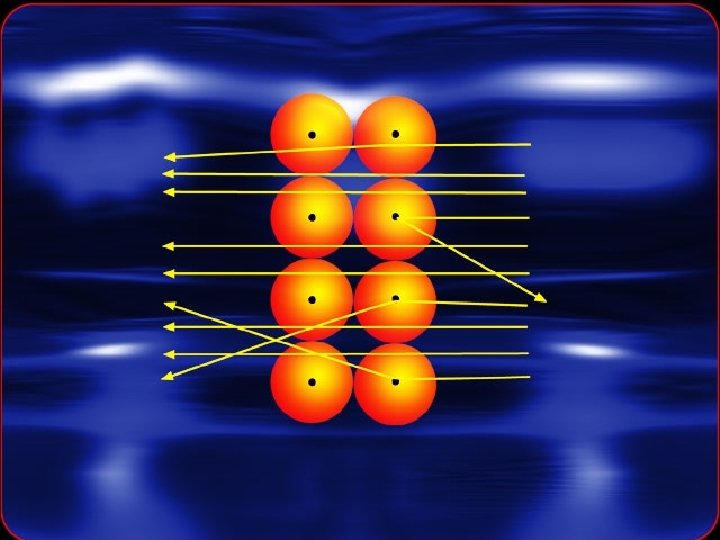
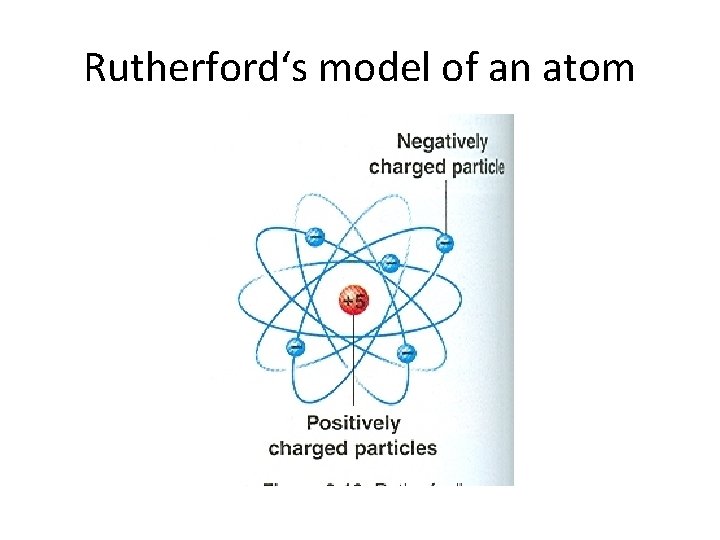
Rutherford's Model • In 1911, a British scientist named Ernest Rutherford discovered that an atom is mostly empty space. • He concluded that the positively charged particles are contained in a small central core called the nucleus. • He also concluded that negatively charged particles were attracted to positively charged particles found in the nucleus. • This attraction holds the negatively charged particles in the atom.



Rutherford-1911 Not empty space • Discovered the nucleus. • Named the positively charged particles protons. • Said that the atom was mostly empty space and electrons traveled around the nucleus.

Rutherford‘s model of an atom

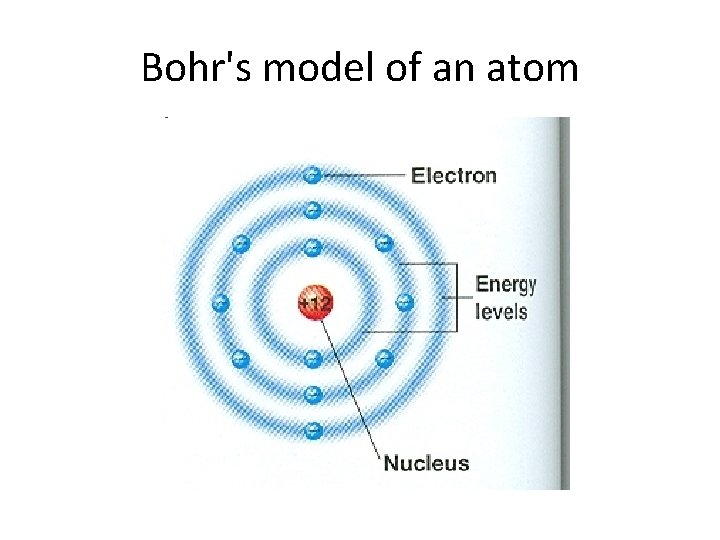
Bohr's Model • Rutherford's model of the atom did not explain the arrangement of electrons. • In 1913, Niels Bohr proposed that the electrons in an atom are found in different energy levels. • Each energy level is at a certain distance from the nucleus. • Electrons in different energy levels move around the nucleus in different orbits. • Bohr's model explains simple atoms such as oxygen well, but it does not explain more complex atoms.

pt m e t t a y M g is at smilin a lower evel. l y g r e n e Niels Bohr-1913 • FOUND EVIDENCE THAT ELECTRONS WERE ARRANGED IN ENERGY LEVELS.

Bohr's model of an atom

Gilbert N. Lewis - 1916 • Developed dot structure diagram of atoms to explain how they bond together. • Published works on how atoms combine or “bond”.

Chadwick & the Nucleus • When chemists started looking into how much mass an atom has, they noticed that there is a mysterious source that they couldn’t figure out that made the mass bigger than they thought. • James Chadwick hypothesized and tested to prove that there is another particle in the nucleus. • He found that these particles have no positive or negative charge.

CHADWICK-1932 • DISCOVERED THE NEUTRON. • His ideas allowed for man-made elements to be created.

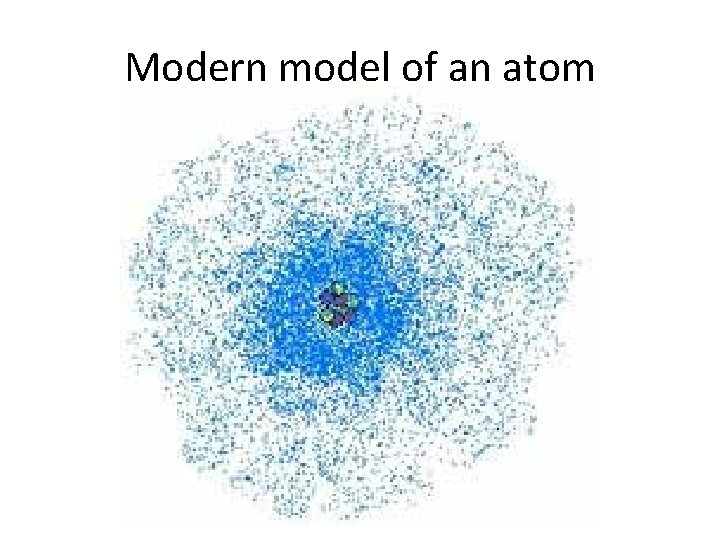
Modern Model • According to the modern model, the location of the electrons in an atom cannot be known. • Therefore, the modern model of the atom does not show any paths that electrons could be found in. • Instead, energy levels are used to predict the place where an electron is most likely to be found outside of the nucleus. • This area is called the electron cloud.

Modern model of an atom

The History of Atomic Theory

Movie Made with Atoms! • Regular microscopes cannot give you a visual of an atom using light to see it. • Special electromagnetic lenses in special machines can produce an image of an atom’s nucleus magnified millions and millions of times. • With this technology, scientists can manipulate the location of nuclei to produce images in sequences that look like a movie. • You cannot see any electrons. They are 1836 times smaller than a proton. http: //www. youtube. com/watch? v=o. SCX 78 -8 -q 0