Matter and Atomic Structure Matter and Atomic Structure
- Slides: 13

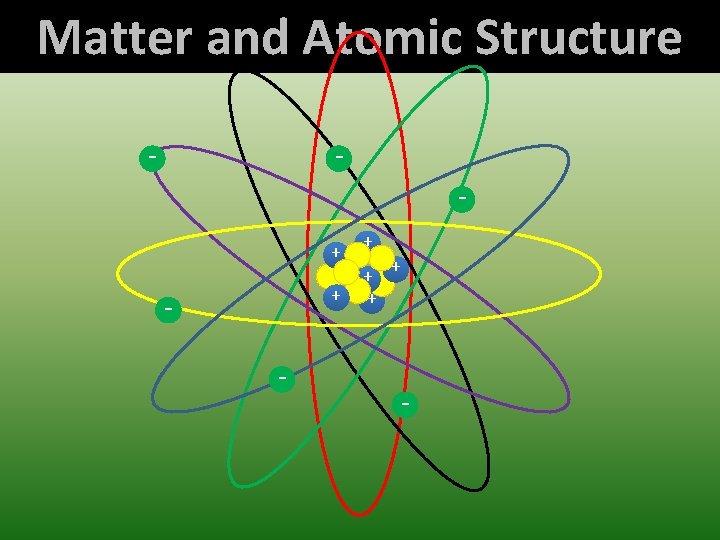
Matter and Atomic Structure - + + + + -

Matter and Atomic Structure Objectives: • Describe the sub-atomic particles • Describe the structure of atoms • Define the concept of isotopes

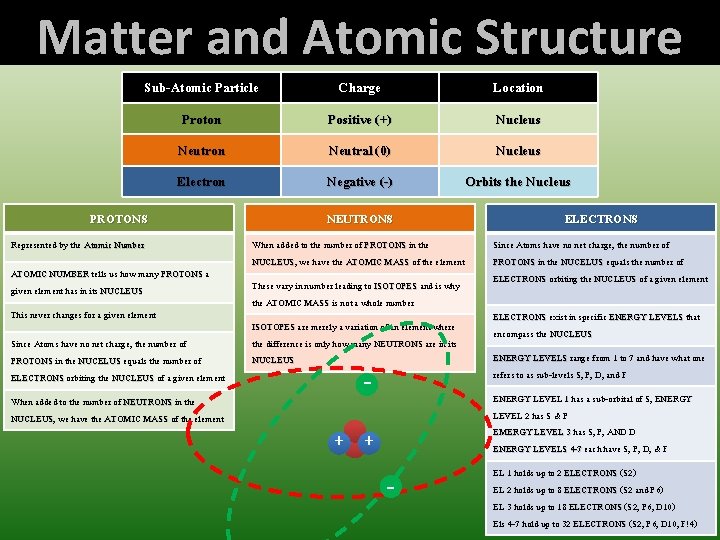
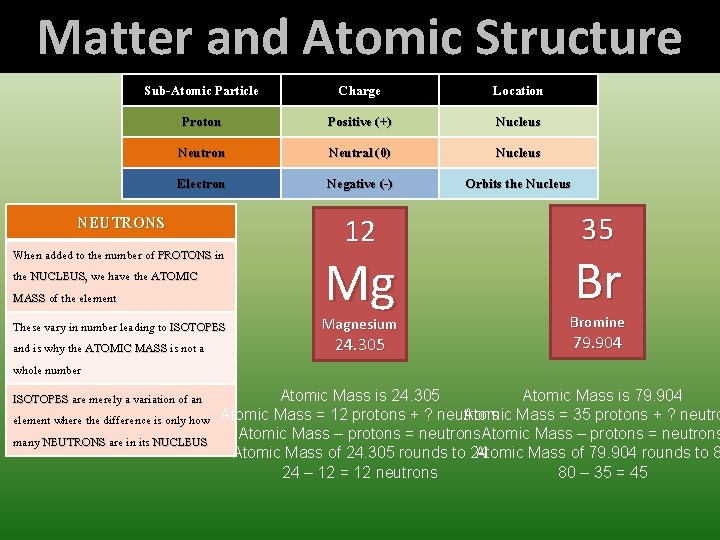
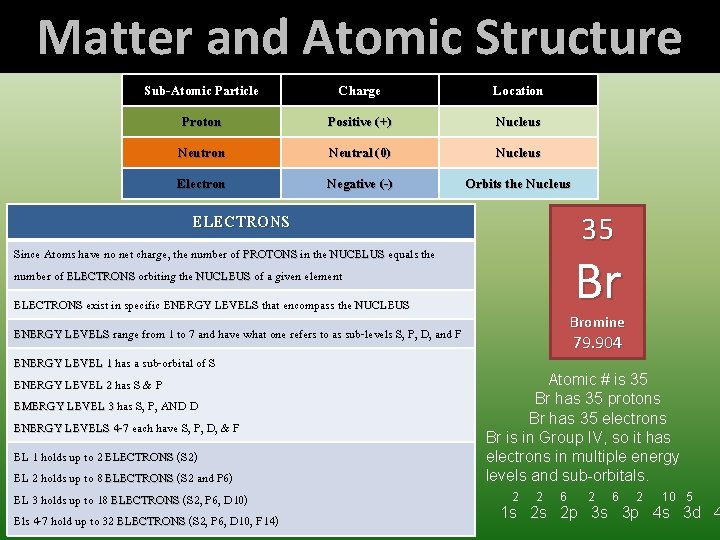
Matter and Atomic Structure Sub-Atomic Particle Charge Location Proton Positive (+) Nucleus Neutron Neutral (0) Nucleus Electron Negative (-) Orbits the Nucleus Represented by the Atomic Number ATOMIC NUMBER tells us how many PROTONS a given element has in its NUCLEUS This never changes for a given element Since Atoms have no net charge, the number of PROTONS in the NUCELUS equals the number of ELECTRONS orbiting the NUCLEUS of a given element When added to the number of NEUTRONS in the NUCLEUS, NUCLEUS we have the ATOMIC MASS of the element NEUTRONS ELECTRONS When added to the number of PROTONS in the NUCLEUS, NUCLEUS we have the ATOMIC MASS of the element Since Atoms have no net charge, the number of PROTONS in the NUCELUS equals the number of ELECTRONS orbiting the NUCLEUS of a given element These vary in number leading to ISOTOPES and is why the ATOMIC MASS is not a whole number ISOTOPES are merely a variation of an element where the difference is only how many NEUTRONS are in its NUCLEUS - + ELECTRONS exist in specific ENERGY LEVELS that encompass the NUCLEUS ENERGY LEVELS range from 1 to 7 and have what one refers to as sub-levels S, P, D, and F ENERGY LEVEL 1 has a sub-orbital of S, ENERGY LEVEL 2 has S & P EMERGY LEVEL 3 has S, P, AND D ENERGY LEVELS 4 -7 each have S, P, D, & F + - PROTONS EL 1 holds up to 2 ELECTRONS (S 2) EL 2 holds up to 8 ELECTRONS (S 2 and P 6) EL 3 holds up to 18 ELECTRONS (S 2, P 6, D 10) Els 4 -7 hold up to 32 ELECTRONS (S 2, P 6, D 10, F!4)

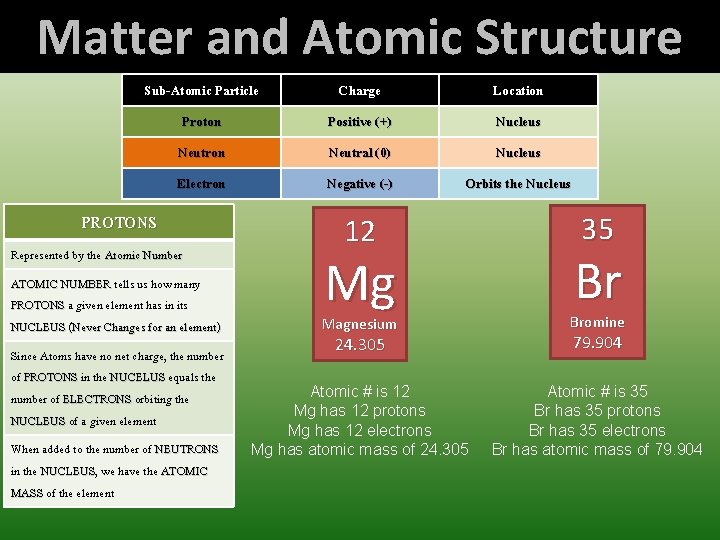
Matter and Atomic Structure Sub-Atomic Particle Charge Location Proton Positive (+) Nucleus Neutron Neutral (0) Nucleus Electron Negative (-) Orbits the Nucleus PROTONS Represented by the Atomic Number ATOMIC NUMBER tells us how many PROTONS a given element has in its NUCLEUS (Never Changes for an element) Since Atoms have no net charge, the number of PROTONS in the NUCELUS equals the number of ELECTRONS orbiting the NUCLEUS of a given element When added to the number of NEUTRONS in the NUCLEUS, NUCLEUS we have the ATOMIC MASS of the element 12 Mg 35 Br Magnesium Bromine 24. 305 79. 904 Atomic # is 12 Mg has 12 protons Mg has 12 electrons Mg has atomic mass of 24. 305 Atomic # is 35 Br has 35 protons Br has 35 electrons Br has atomic mass of 79. 904

Matter and Atomic Structure Sub-Atomic Particle Charge Location Proton Positive (+) Nucleus Neutron Neutral (0) Nucleus Electron Negative (-) Orbits the Nucleus NEUTRONS When added to the number of PROTONS in the NUCLEUS, NUCLEUS we have the ATOMIC MASS of the element These vary in number leading to ISOTOPES and is why the ATOMIC MASS is not a whole number 12 Mg Magnesium 24. 305 35 Br Bromine 79. 904 Atomic Mass is 24. 305 Atomic Mass is 79. 904 ISOTOPES are merely a variation of an Atomic Mass = 35 protons + ? neutro element where the difference is only how Atomic Mass = 12 protons + ? neutrons Atomic Mass – protons = neutrons many NEUTRONS are in its NUCLEUS Atomic Mass of 24. 305 rounds to 24 Atomic Mass of 79. 904 rounds to 8 24 – 12 = 12 neutrons 80 – 35 = 45

Matter and Atomic Structure Sub-Atomic Particle Charge Location Proton Positive (+) Nucleus Neutron Neutral (0) Nucleus Electron Negative (-) Orbits the Nucleus ELECTRONS 35 Since Atoms have no net charge, the number of PROTONS in the NUCELUS equals the number of ELECTRONS orbiting the NUCLEUS of a given element Br ELECTRONS exist in specific ENERGY LEVELS that encompass the NUCLEUS Bromine ENERGY LEVELS range from 1 to 7 and have what one refers to as sub-levels S, P, D, and F ENERGY LEVEL 1 has a sub-orbital of S ENERGY LEVEL 2 has S & P EMERGY LEVEL 3 has S, P, AND D ENERGY LEVELS 4 -7 each have S, P, D, & F EL 1 holds up to 2 ELECTRONS (S 2) EL 2 holds up to 8 ELECTRONS (S 2 and P 6) EL 3 holds up to 18 ELECTRONS (S 2, P 6, D 10) Els 4 -7 hold up to 32 ELECTRONS (S 2, P 6, D 10, F 14) 79. 904 Atomic # is 35 Br has 35 protons Br has 35 electrons Br is in Group IV, so it has electrons in multiple energy levels and sub-orbitals. 2 2 6 2 10 5 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4

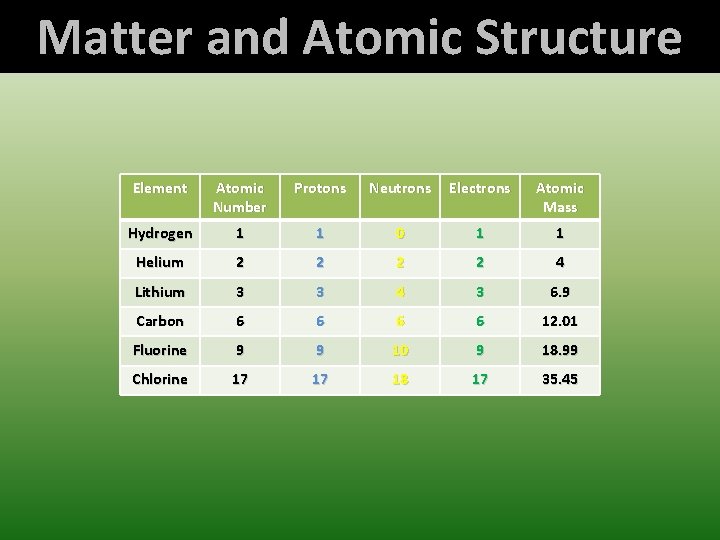
Matter and Atomic Structure Element Atomic Number Protons Neutrons Electrons Atomic Mass Number Mass Hydrogen 1 1 0 1 1 Helium 2 2 4 Lithium 3 3 4 3 6. 9 Carbon 6 6 12. 01 Fluorine 9 9 10 9 18. 99 Chlorine 17 17 18 17 35. 45

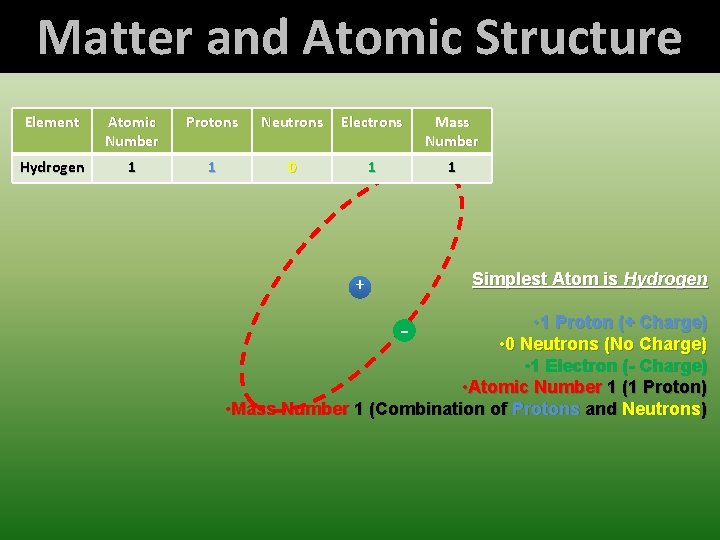
Matter and Atomic Structure Element Atomic Number Protons Neutrons Electrons Mass Number Hydrogen 1 1 0 1 1 Simplest Atom is Hydrogen + • 1 Proton (+ Charge) • 0 Neutrons (No Charge) • 1 Electron (- Charge) • Atomic Number 1 (1 Proton) • Mass Number 1 (Combination of Protons and Neutrons) -

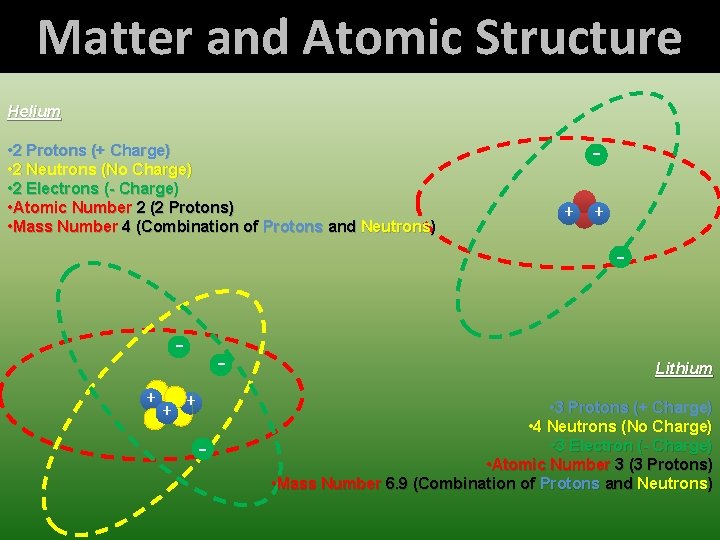
Matter and Atomic Structure Helium • 2 Protons (+ Charge) • 2 Neutrons (No Charge) • 2 Electrons (- Charge) • Atomic Number 2 (2 Protons) • Mass Number 4 (Combination of Protons and Neutrons) + + - + - Lithium • 3 Protons (+ Charge) • 4 Neutrons (No Charge) • 3 Electron (- Charge) • Atomic Number 3 (3 Protons) • Mass Number 6. 9 (Combination of Protons and Neutrons)

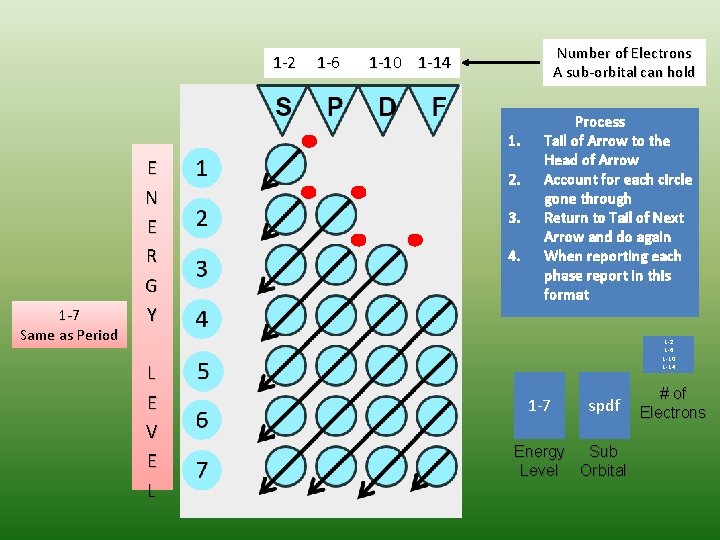
1 -2 1 -6 Number of Electrons A sub-orbital can hold 1 -10 1 -14 1. 1 -7 Same as Period E N E R G Y L E V E L 2. 3. 4. Process Tail of Arrow to the Head of Arrow Account for each circle gone through Return to Tail of Next Arrow and do again When reporting each phase report in this format 1 -2 1 -6 1 -10 1 -14 1 -7 spdf Energy Sub Level Orbital # of Electrons

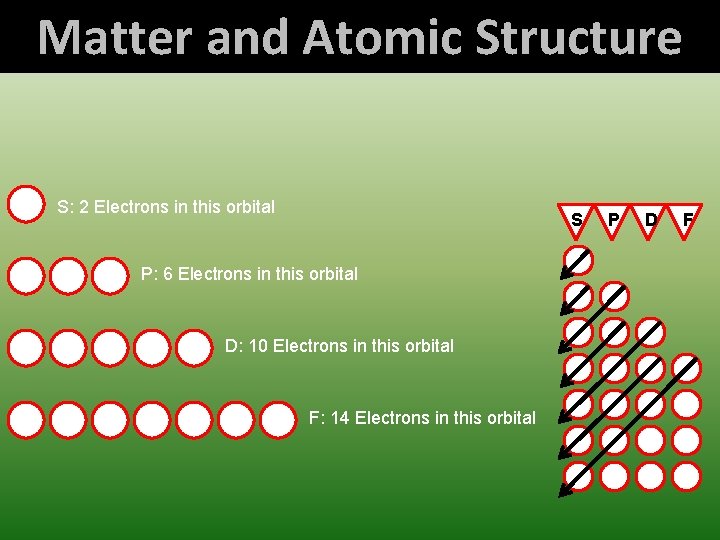
Matter and Atomic Structure S: 2 Electrons in this orbital S P: 6 Electrons in this orbital D: 10 Electrons in this orbital F: 14 Electrons in this orbital P D F

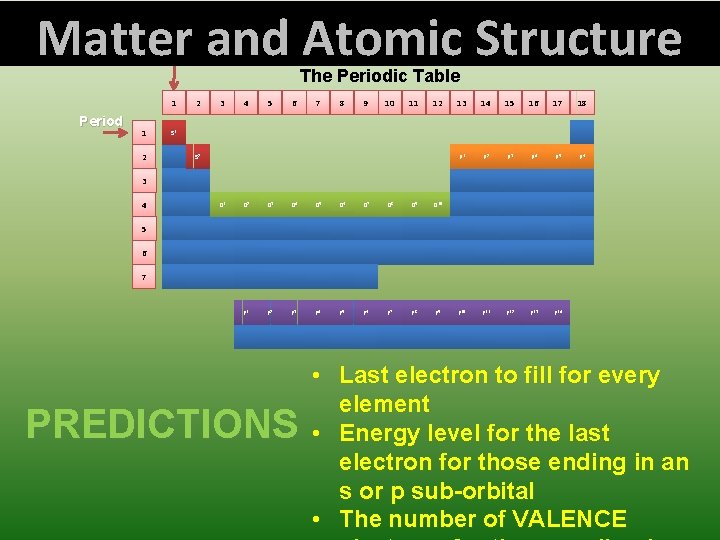
Matter and Atomic Structure Group The Periodic Table 1 Period 1 2 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 P 1 P 2 P 3 P 4 P 5 P 6 F 10 F 11 F 12 F 13 F 14 S 1 S 2 3 4 D 1 D 2 D 3 D 4 D 5 D 6 D 7 D 8 D 9 D 10 F 1 F 2 F 3 F 4 F 5 F 6 F 7 F 8 F 9 5 6 7 PREDICTIONS • Last electron to fill for every element • Energy level for the last electron for those ending in an s or p sub-orbital • The number of VALENCE

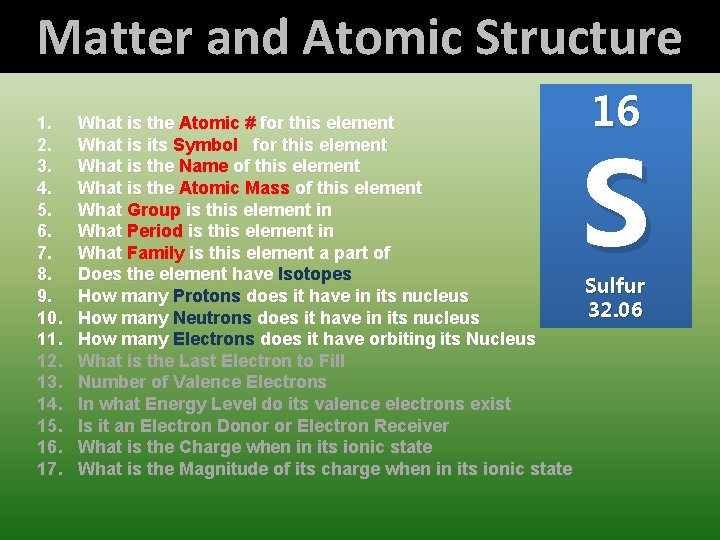
Matter and Atomic Structure 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 16 What is the Atomic # for this element What is its Symbol for this element What is the Name of this element What is the Atomic Mass of this element What Group is this element in What Period is this element in What Family is this element a part of Does the element have Isotopes Sulfur How many Protons does it have in its nucleus 32. 06 How many Neutrons does it have in its nucleus How many Electrons does it have orbiting its Nucleus What is the Last Electron to Fill Number of Valence Electrons In what Energy Level do its valence electrons exist Is it an Electron Donor or Electron Receiver What is the Charge when in its ionic state What is the Magnitude of its charge when in its ionic state S