ATOMIC STRUCTURE Atomic Structure All matter is composed









- Slides: 43

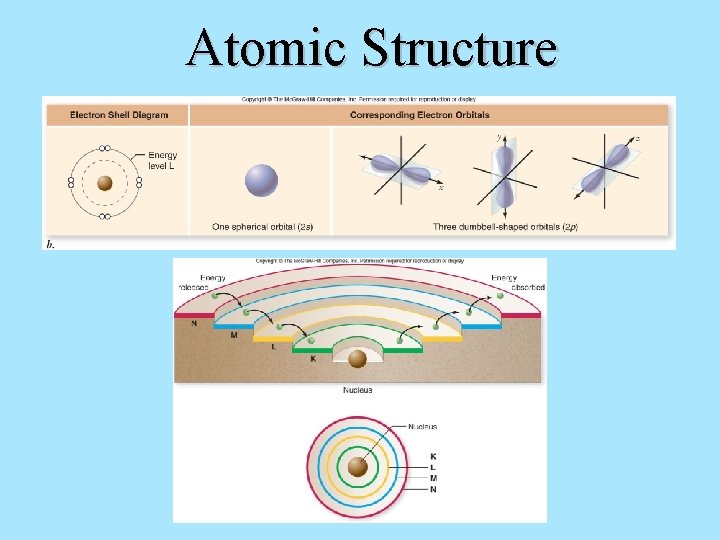
ATOMIC STRUCTURE

Atomic Structure All matter is composed of atoms. Understanding the structure of atoms is critical to understanding the properties of matter

HISTORY OF THE ATOM 1808 John Dalton suggested that all matter was made up of tiny spheres that were able to bounce around with perfect elasticity and called them ATOMS

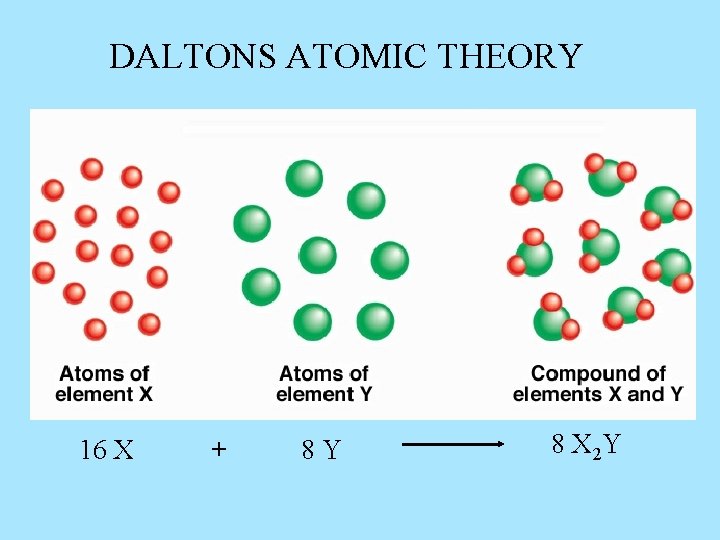
DALTONS ATOMIC THEORY 16 X + 8 Y 8 X 2 Y

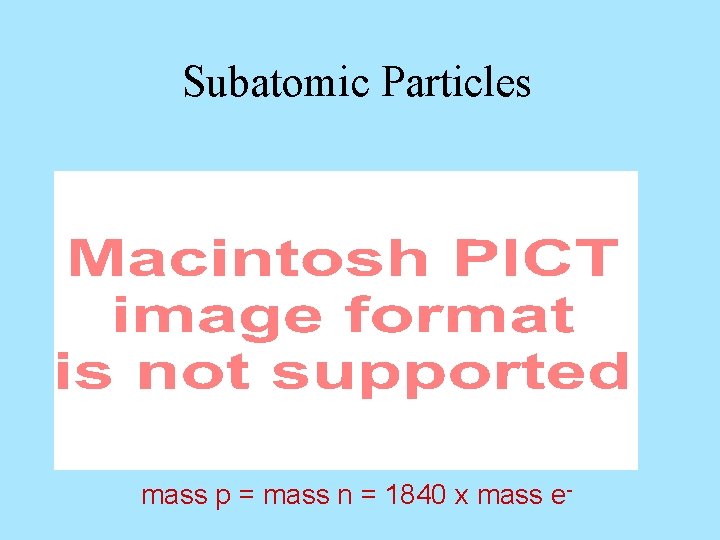
Subatomic Particles mass p = mass n = 1840 x mass e-

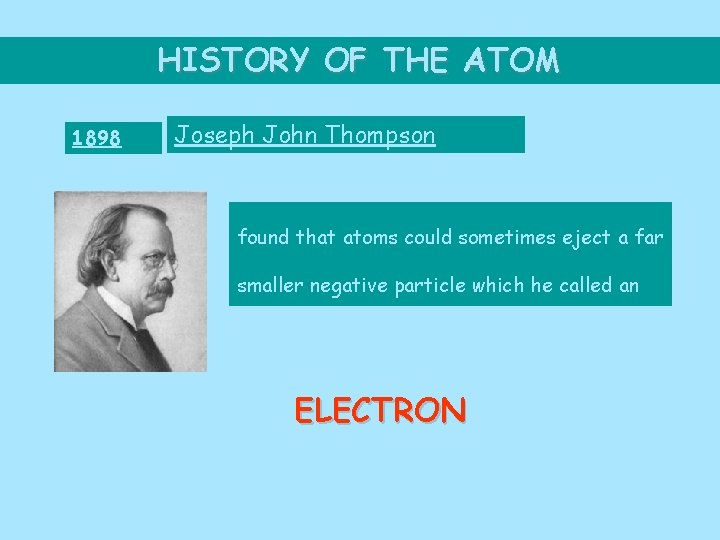
HISTORY OF THE ATOM 1898 Joseph John Thompson found that atoms could sometimes eject a far smaller negative particle which he called an ELECTRON

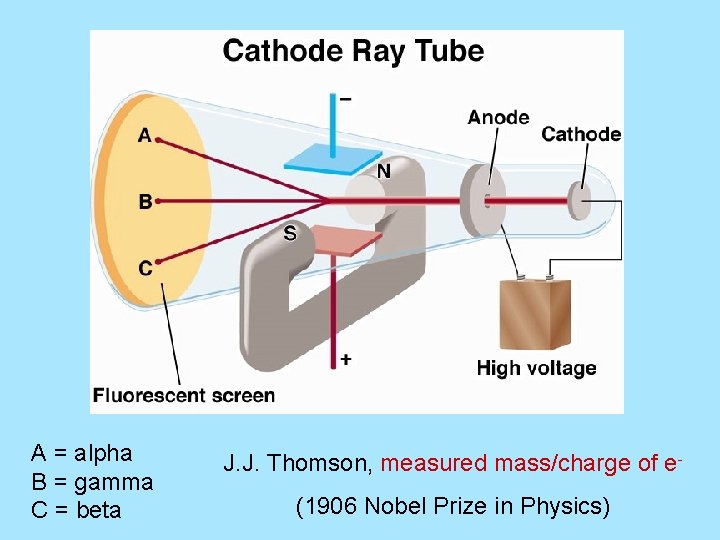
A = alpha B = gamma C = beta J. J. Thomson, measured mass/charge of e(1906 Nobel Prize in Physics)

CHARGE OF AN ELECTRON gold foil helium nuclei Millikan oil drop experiment

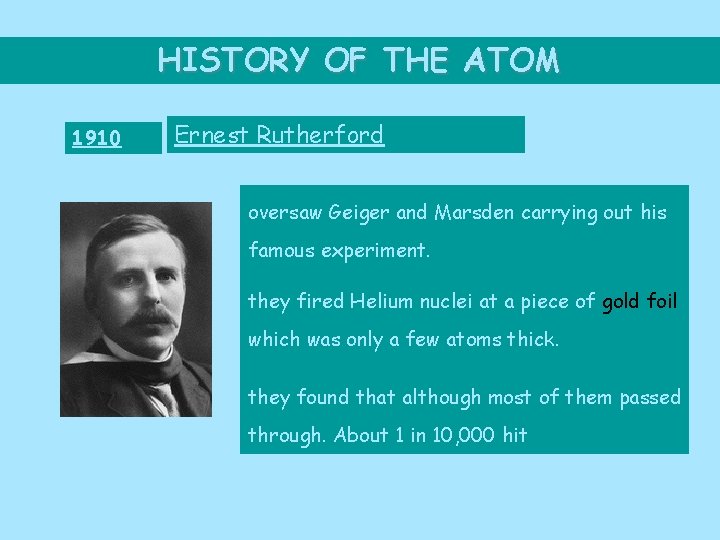
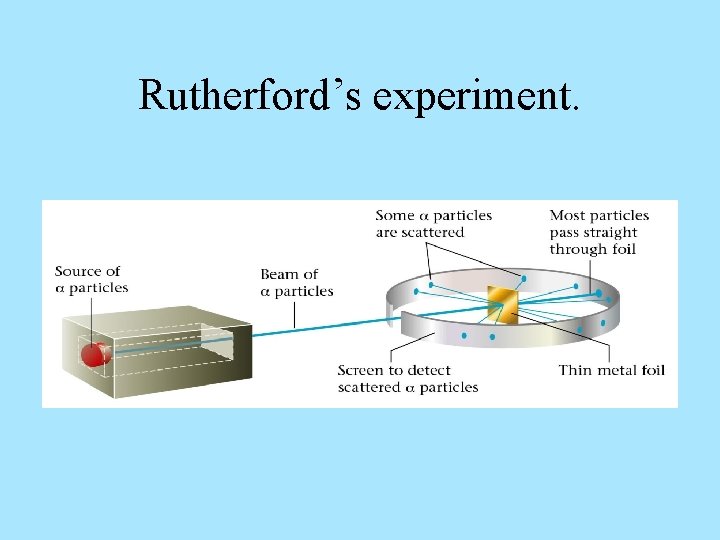
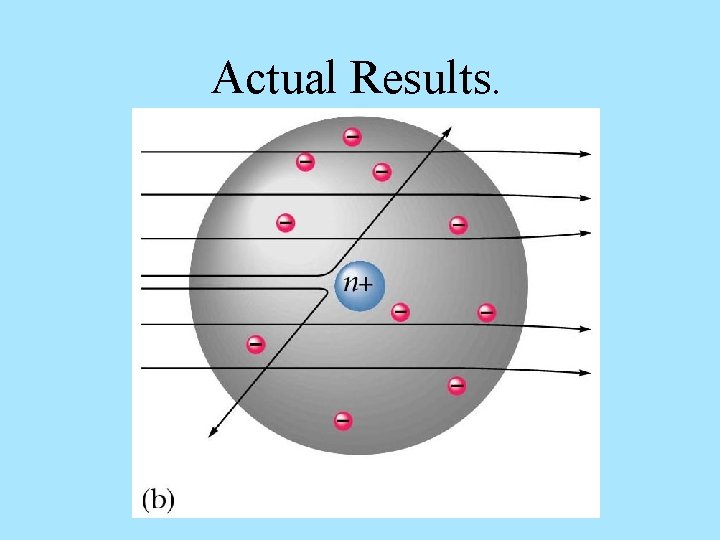
HISTORY OF THE ATOM 1910 Ernest Rutherford oversaw Geiger and Marsden carrying out his famous experiment. they fired Helium nuclei at a piece of gold foil which was only a few atoms thick. they found that although most of them passed through. About 1 in 10, 000 hit

Rutherford’s experiment.

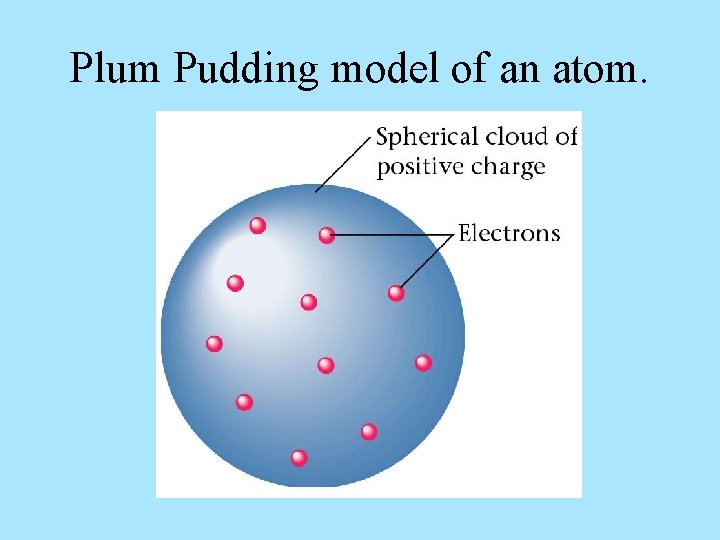
Plum Pudding model of an atom.

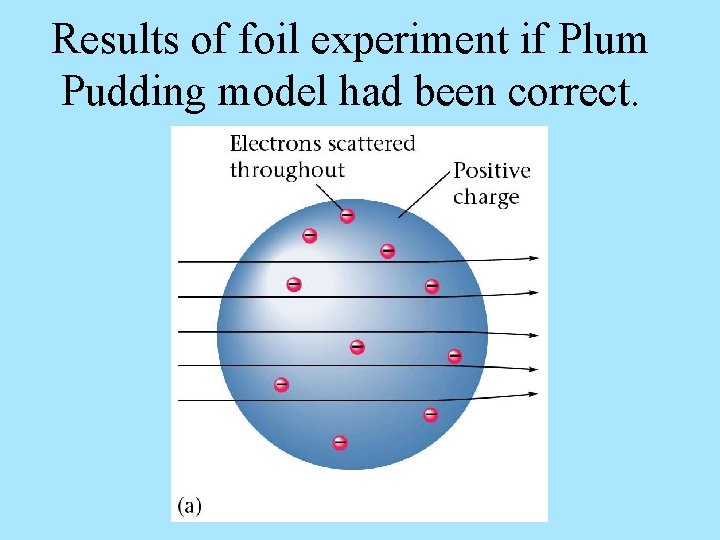
Results of foil experiment if Plum Pudding model had been correct.

Actual Results.

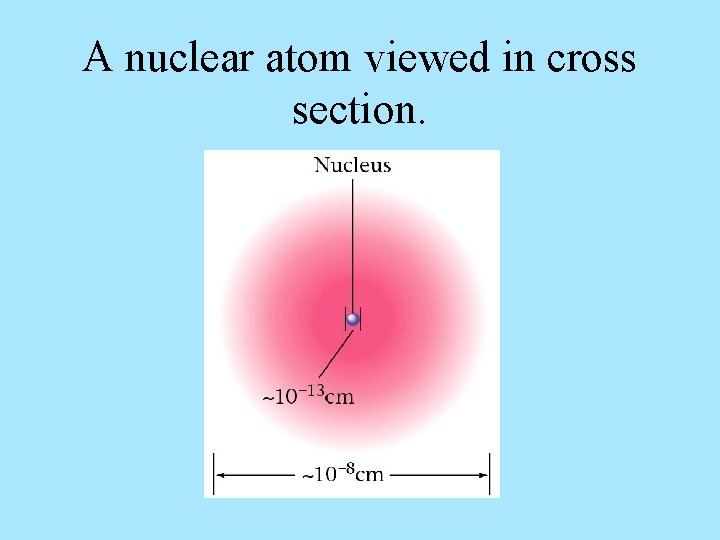
A nuclear atom viewed in cross section.

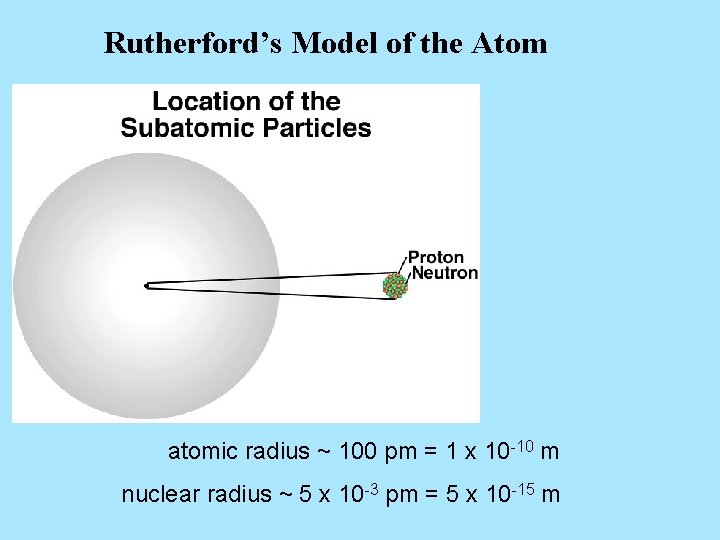
Rutherford’s Model of the Atom atomic radius ~ 100 pm = 1 x 10 -10 m nuclear radius ~ 5 x 10 -3 pm = 5 x 10 -15 m

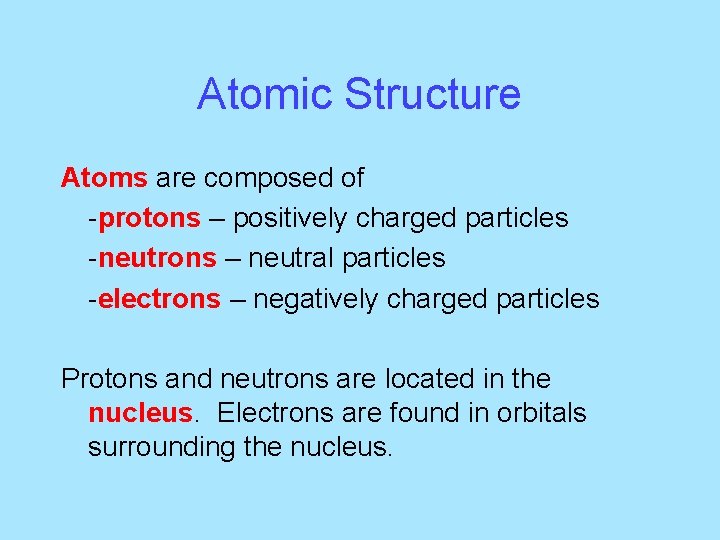
Atomic Structure Atoms are composed of -protons – positively charged particles -neutrons – neutral particles -electrons – negatively charged particles Protons and neutrons are located in the nucleus. Electrons are found in orbitals surrounding the nucleus.

HELIUM ATOM Shell proton + electron N N + - neutron

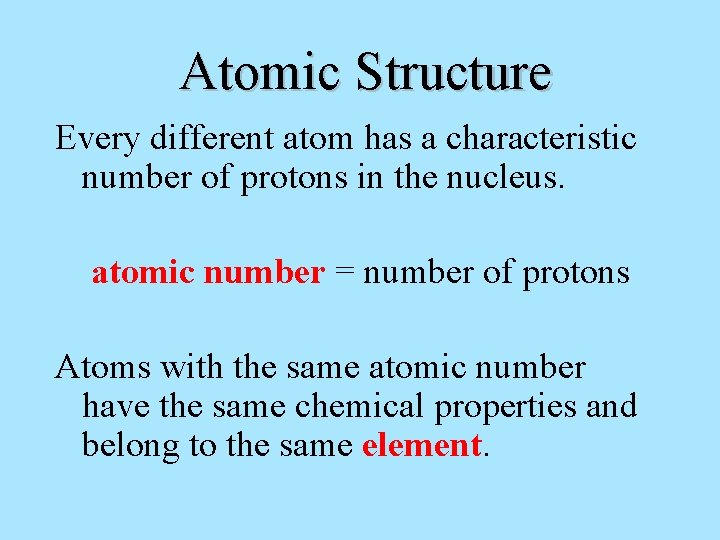
Atomic Structure Every different atom has a characteristic number of protons in the nucleus. atomic number = number of protons Atoms with the same atomic number have the same chemical properties and belong to the same element.

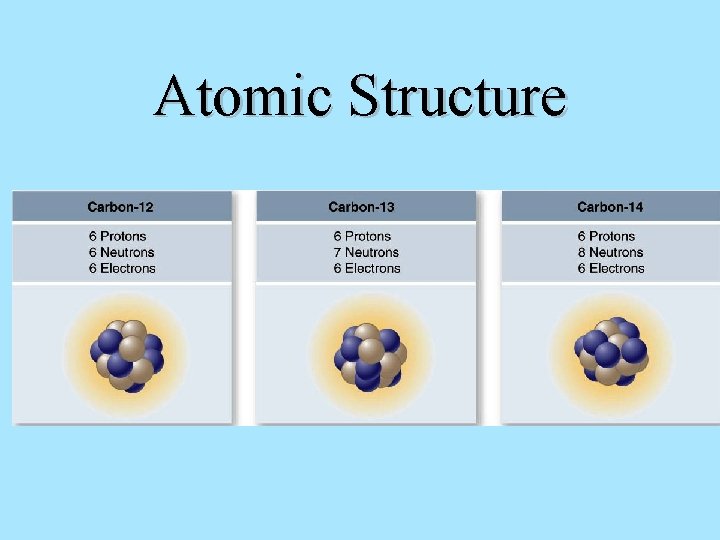
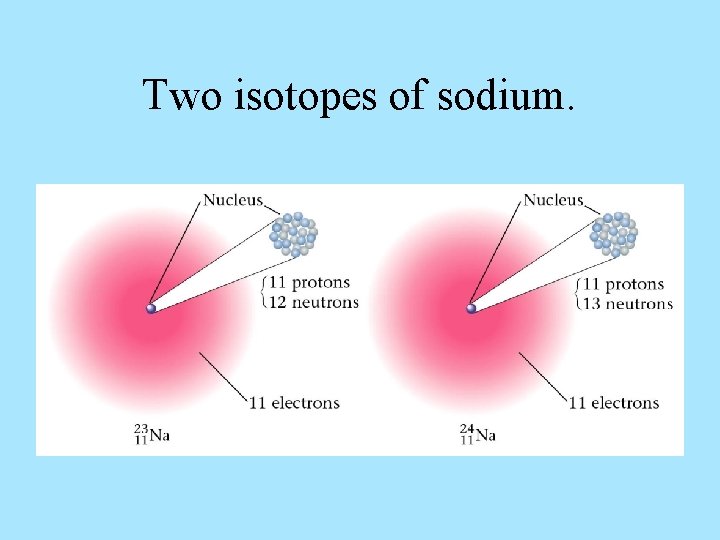
Atomic Structure Each proton and neutron has a mass of approximately 1 dalton. The sum of protons and neutrons is the atom’s atomic mass. Isotopes – atoms of the same element that have different atomic mass numbers due to different numbers of neutrons.

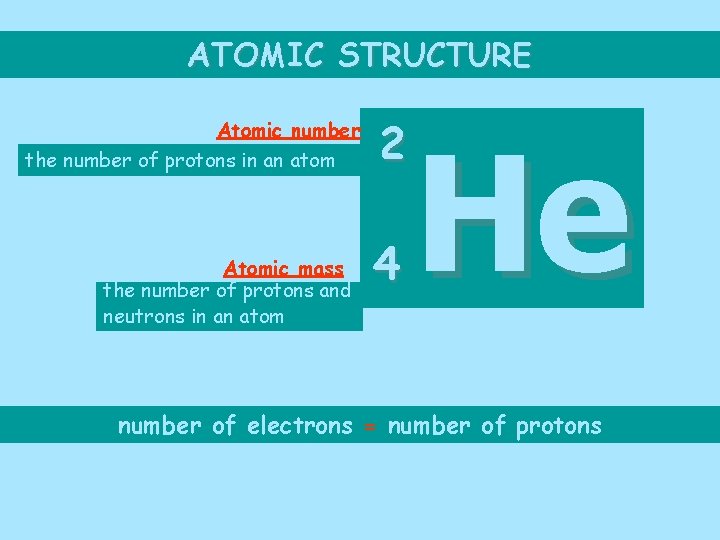
ATOMIC STRUCTURE Atomic number the number of protons in an atom Atomic mass the number of protons and neutrons in an atom 2 4 He number of electrons = number of protons

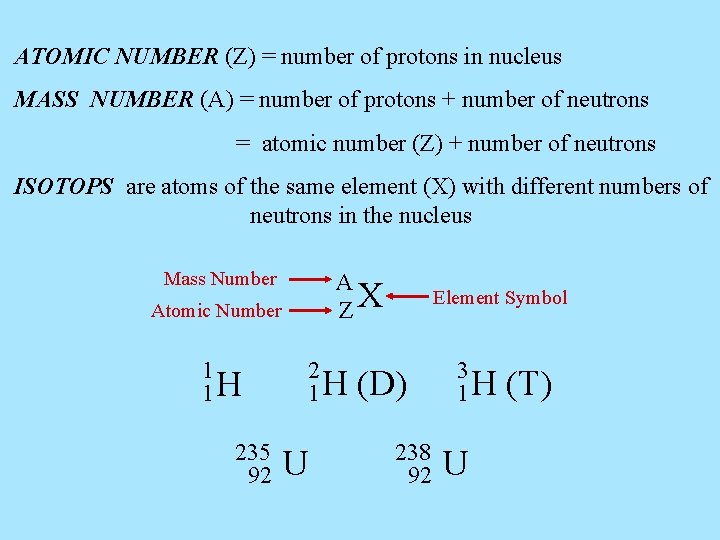
ATOMIC NUMBER (Z) = number of protons in nucleus MASS NUMBER (A) = number of protons + number of neutrons = atomic number (Z) + number of neutrons ISOTOPS are atoms of the same element (X) with different numbers of neutrons in the nucleus Mass Number A ZX Atomic Number 1 1 H 235 92 2 1 H U Element Symbol (D) 238 92 3 1 H U (T)

Atomic Structure

Atomic Structure

Two isotopes of sodium.

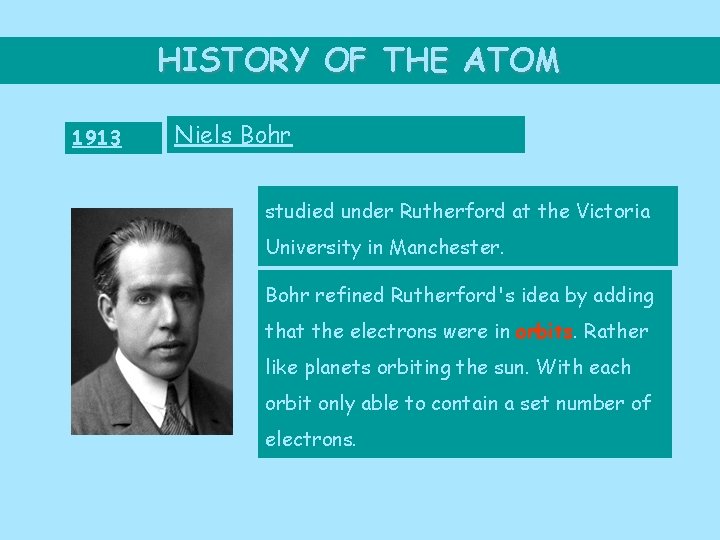
HISTORY OF THE ATOM 1913 Niels Bohr studied under Rutherford at the Victoria University in Manchester. Bohr refined Rutherford's idea by adding that the electrons were in orbits. Rather like planets orbiting the sun. With each orbit only able to contain a set number of electrons.

MULTIELECTRON ATOMS


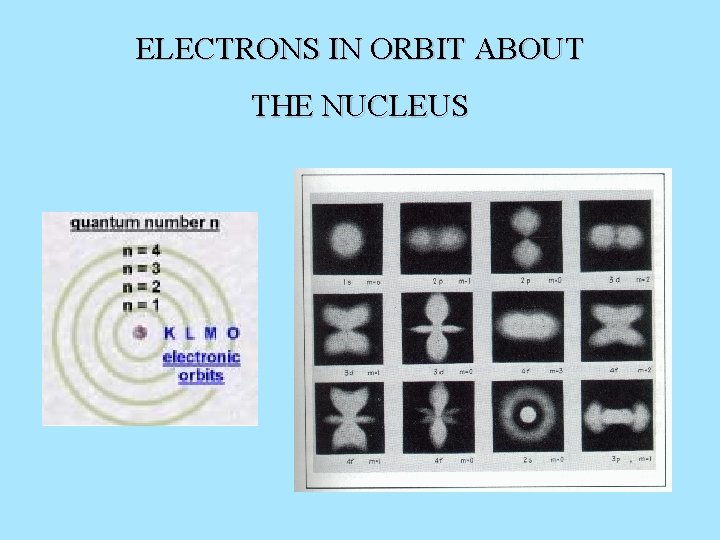
ELECTRONS IN ORBIT ABOUT THE NUCLEUS

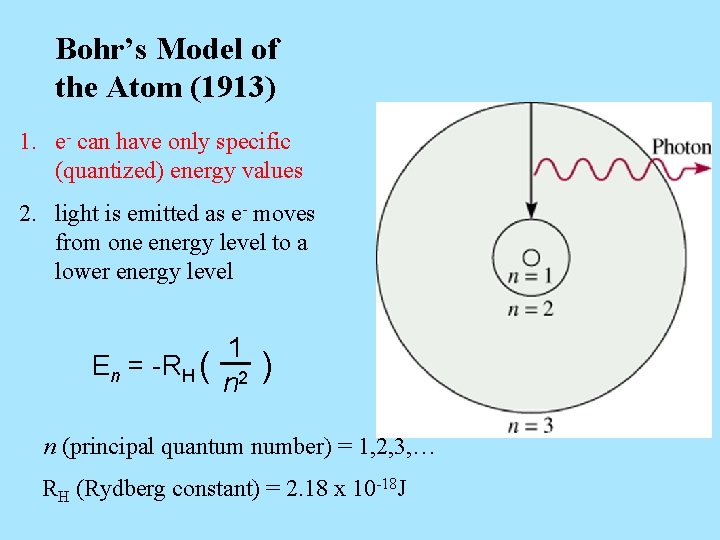
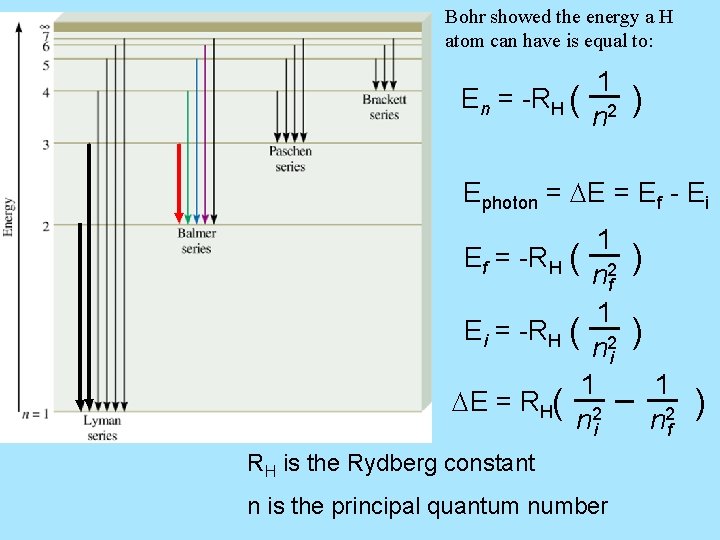
Bohr’s Model of the Atom (1913) 1. e- can have only specific (quantized) energy values 2. light is emitted as e- moves from one energy level to a lower energy level En = -RH ( 1 n 2 ) n (principal quantum number) = 1, 2, 3, … RH (Rydberg constant) = 2. 18 x 10 -18 J

The Bohr Model of the Atom

Atomic Structure

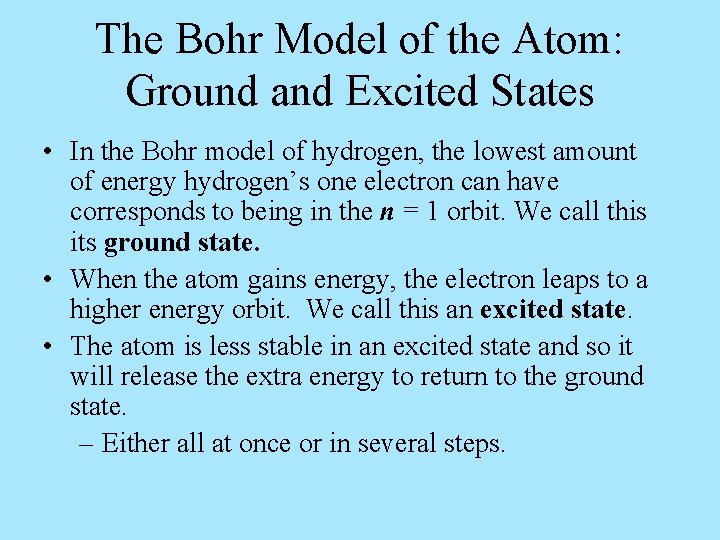
The Bohr Model of the Atom: Ground and Excited States • In the Bohr model of hydrogen, the lowest amount of energy hydrogen’s one electron can have corresponds to being in the n = 1 orbit. We call this its ground state. • When the atom gains energy, the electron leaps to a higher energy orbit. We call this an excited state. • The atom is less stable in an excited state and so it will release the extra energy to return to the ground state. – Either all at once or in several steps.



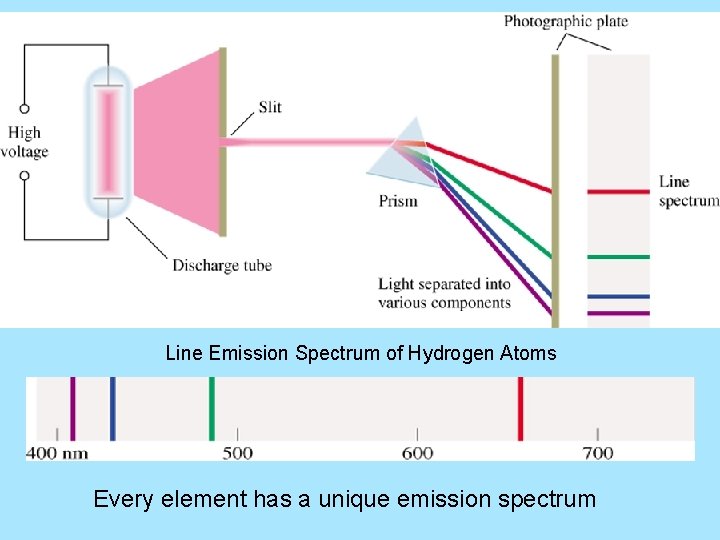
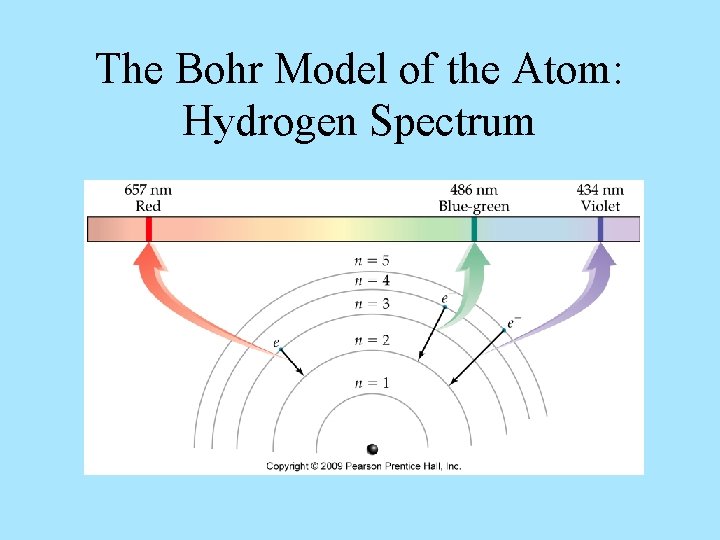
Line Emission Spectrum of Hydrogen Atoms Every element has a unique emission spectrum

The Bohr Model of the Atom: Hydrogen Spectrum • Every hydrogen atom has identical orbits, so every hydrogen atom can undergo the same energy transitions. • However, since the distances between the orbits in an atom are not all the same, no two leaps in an atom will have the same energy. – The closer the orbits are in energy, the lower the energy of the photon emitted. – Lower energy photon = longer wavelength. • Therefore, we get an emission spectrum that has a lot of lines that are unique to hydrogen.


The Bohr Model of the Atom: Hydrogen Spectrum

Bohr showed the energy a H atom can have is equal to: En = -RH ( 1 n 2 ) Ephoton = DE = Ef - Ei 1 Ef = -RH ( 2 nf 1 Ei = -RH ( 2 ni 1 DE = RH( 2 ni RH is the Rydberg constant n is the principal quantum number ) ) 1 n 2 f )

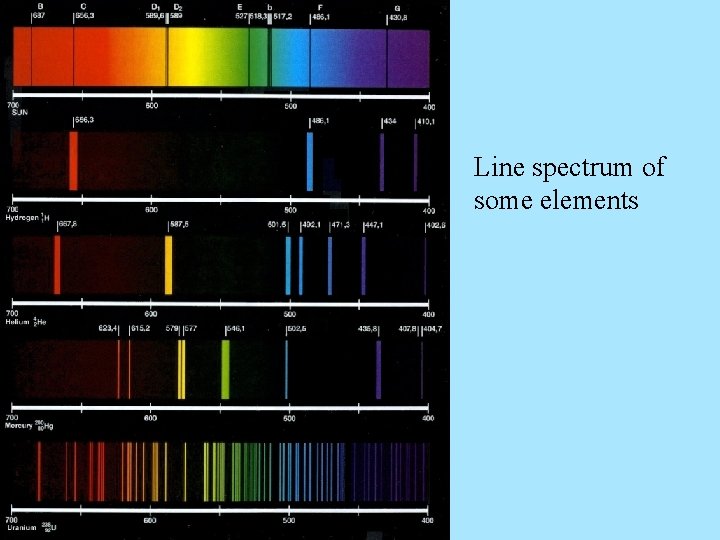
Line spectrum of some elements

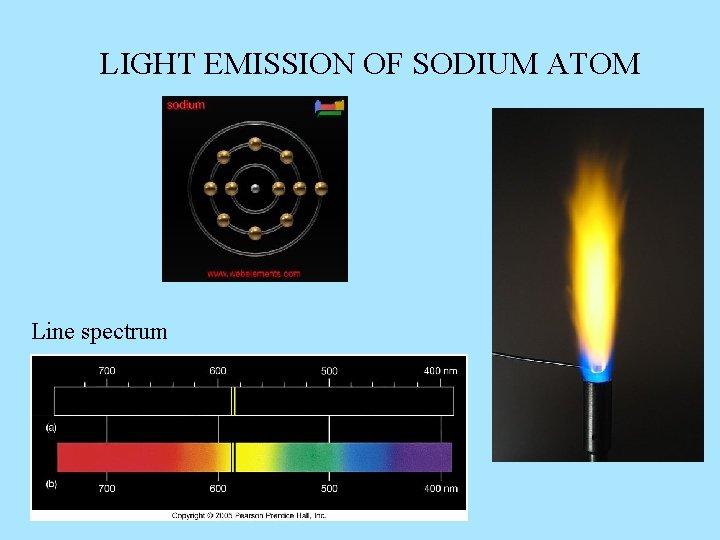
LIGHT EMISSION OF SODIUM ATOM Line spectrum

Atomic Structure Neutral atoms have the same number of protons and electrons. Ions are charged atoms. -cations – have more protons than electrons and are positively charged -anions – have more electrons than protons and are negatively charged

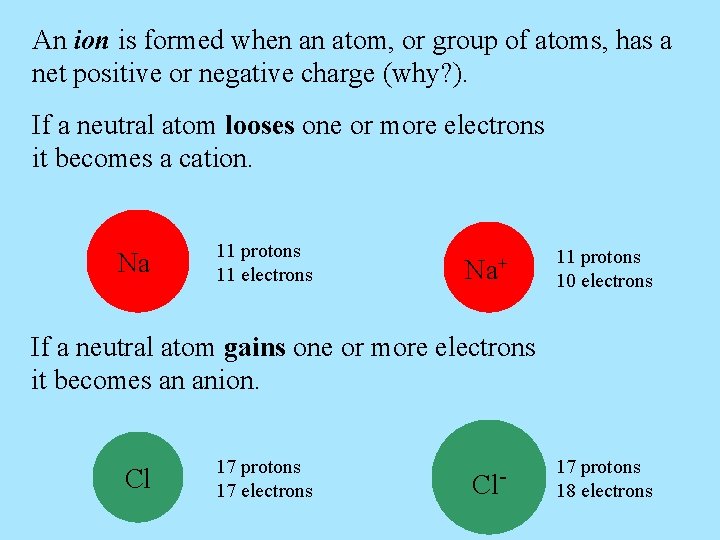
An ion is formed when an atom, or group of atoms, has a net positive or negative charge (why? ). If a neutral atom looses one or more electrons it becomes a cation. Na 11 protons 11 electrons Na+ 11 protons 10 electrons If a neutral atom gains one or more electrons it becomes an anion. Cl 17 protons 17 electrons Cl- 17 protons 18 electrons