Atomic Mass Unit amu atomic mass unit amu
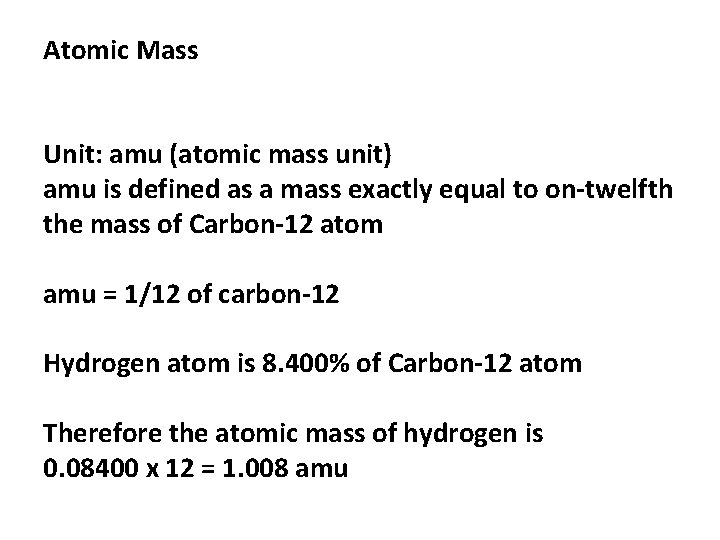


- Slides: 21
Atomic Mass Unit: amu (atomic mass unit) amu is defined as a mass exactly equal to on-twelfth the mass of Carbon-12 atom amu = 1/12 of carbon-12 Hydrogen atom is 8. 400% of Carbon-12 atom Therefore the atomic mass of hydrogen is 0. 08400 x 12 = 1. 008 amu

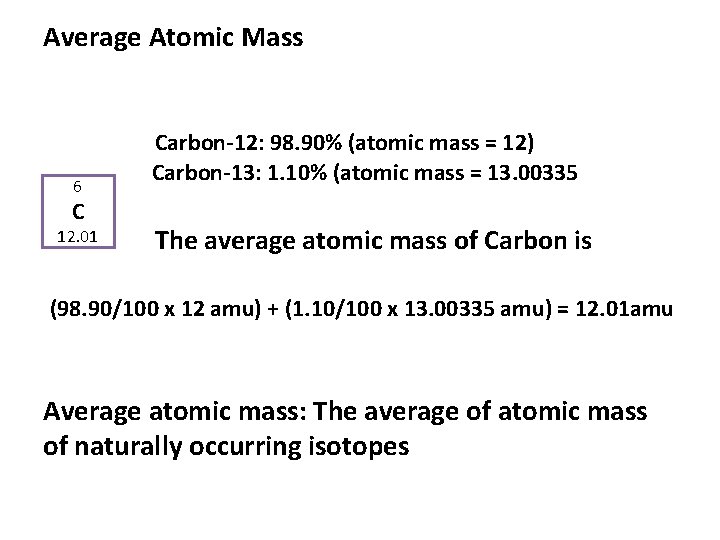
Average Atomic Mass 6 C 12. 01 Carbon-12: 98. 90% (atomic mass = 12) Carbon-13: 1. 10% (atomic mass = 13. 00335 The average atomic mass of Carbon is (98. 90/100 x 12 amu) + (1. 10/100 x 13. 00335 amu) = 12. 01 amu Average atomic mass: The average of atomic mass of naturally occurring isotopes

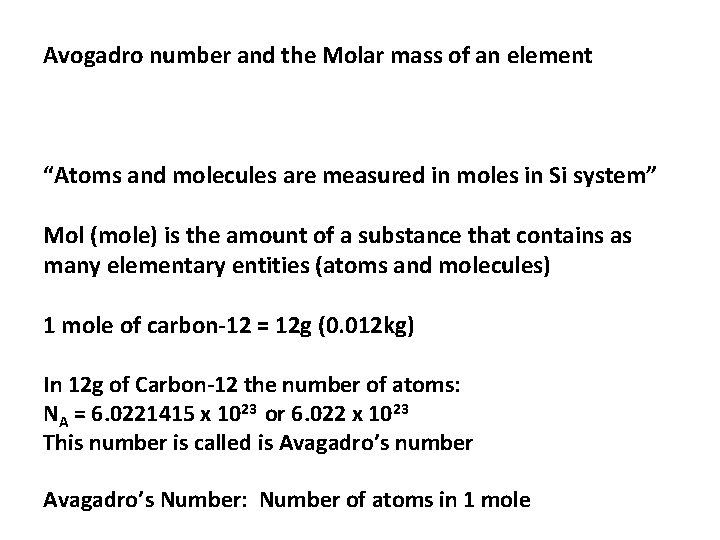
Avogadro number and the Molar mass of an element “Atoms and molecules are measured in moles in Si system” Mol (mole) is the amount of a substance that contains as many elementary entities (atoms and molecules) 1 mole of carbon-12 = 12 g (0. 012 kg) In 12 g of Carbon-12 the number of atoms: NA = 6. 0221415 x 1023 or 6. 022 x 1023 This number is called is Avagadro’s number Avagadro’s Number: Number of atoms in 1 mole

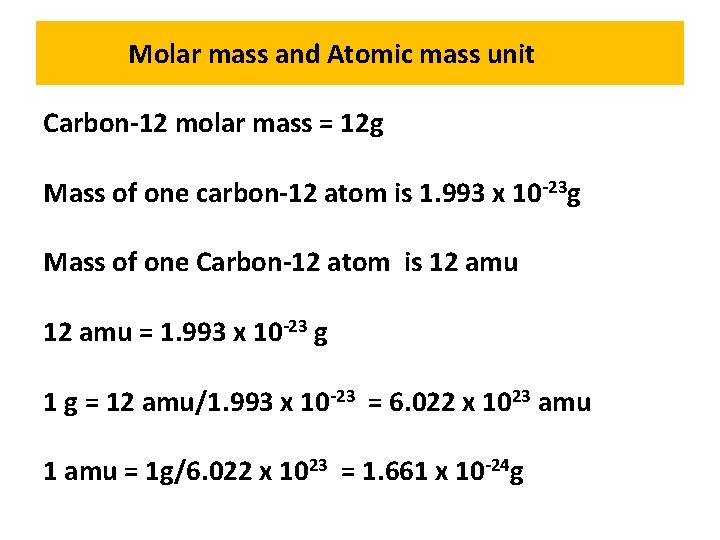
Molar mass and Atomic mass unit Carbon-12 molar mass = 12 g Mass of one carbon-12 atom is 1. 993 x 10 -23 g Mass of one Carbon-12 atom is 12 amu = 1. 993 x 10 -23 g 1 g = 12 amu/1. 993 x 10 -23 = 6. 022 x 1023 amu 1 amu = 1 g/6. 022 x 1023 = 1. 661 x 10 -24 g

Conversion factors for moles & number of atoms 1 mol/molar mass 1 mol/Avogadro’s number How many moles of Helium atoms in 6. 46 g of Helium? 6. 46 g x 1 mol/4. 003 g = 1. 61 mol

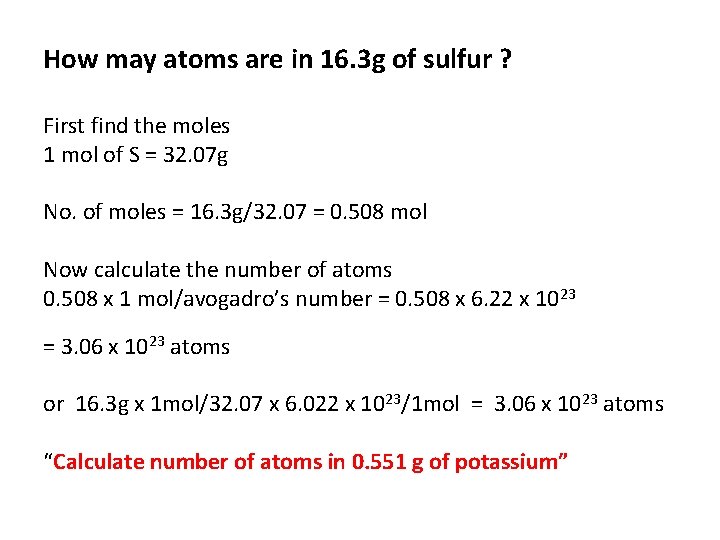
How may atoms are in 16. 3 g of sulfur ? First find the moles 1 mol of S = 32. 07 g No. of moles = 16. 3 g/32. 07 = 0. 508 mol Now calculate the number of atoms 0. 508 x 1 mol/avogadro’s number = 0. 508 x 6. 22 x 1023 = 3. 06 x 1023 atoms or 16. 3 g x 1 mol/32. 07 x 6. 022 x 1023/1 mol = 3. 06 x 1023 atoms “Calculate number of atoms in 0. 551 g of potassium”

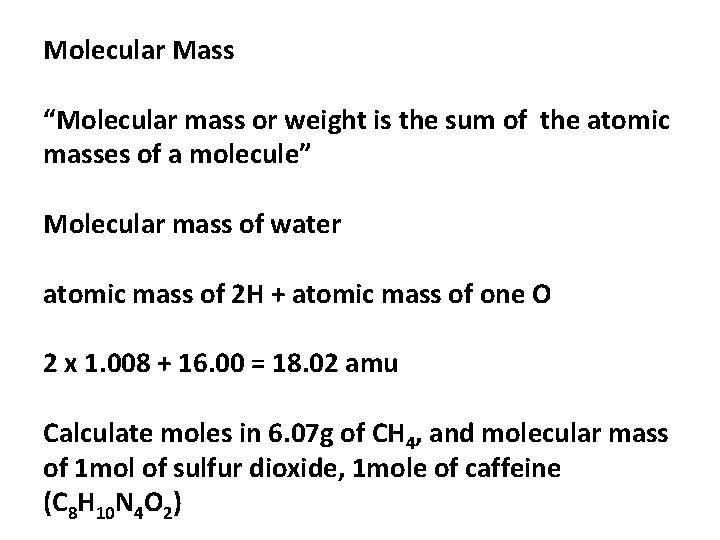
Molecular Mass “Molecular mass or weight is the sum of the atomic masses of a molecule” Molecular mass of water atomic mass of 2 H + atomic mass of one O 2 x 1. 008 + 16. 00 = 18. 02 amu Calculate moles in 6. 07 g of CH 4, and molecular mass of 1 mol of sulfur dioxide, 1 mole of caffeine (C 8 H 10 N 4 O 2)

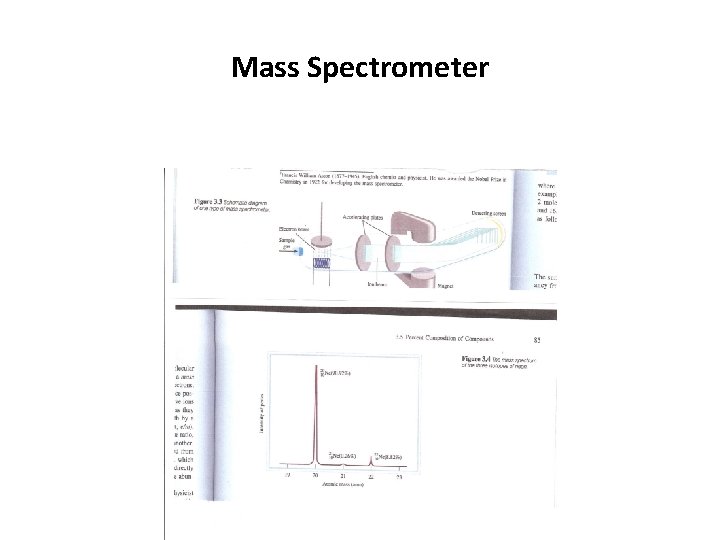
Mass Spectrometer

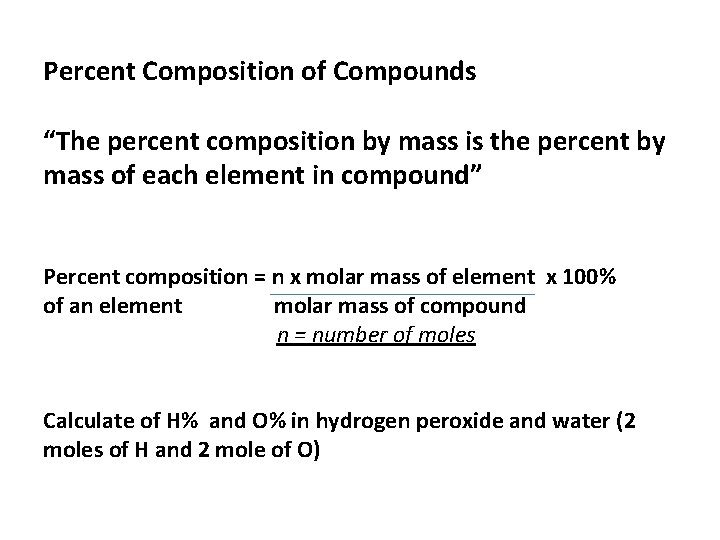
Percent Composition of Compounds “The percent composition by mass is the percent by mass of each element in compound” Percent composition = n x molar mass of element x 100% of an element molar mass of compound n = number of moles Calculate of H% and O% in hydrogen peroxide and water (2 moles of H and 2 mole of O)

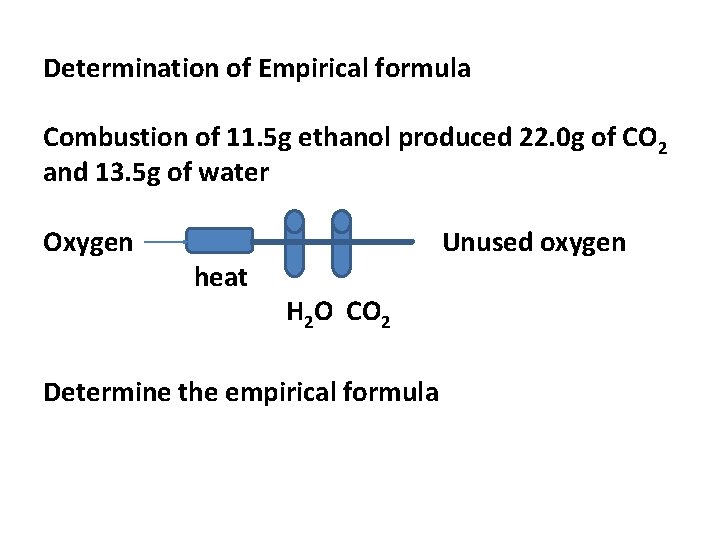
Determination of Empirical formula Combustion of 11. 5 g ethanol produced 22. 0 g of CO 2 and 13. 5 g of water Oxygen heat Unused oxygen H 2 O CO 2 Determine the empirical formula

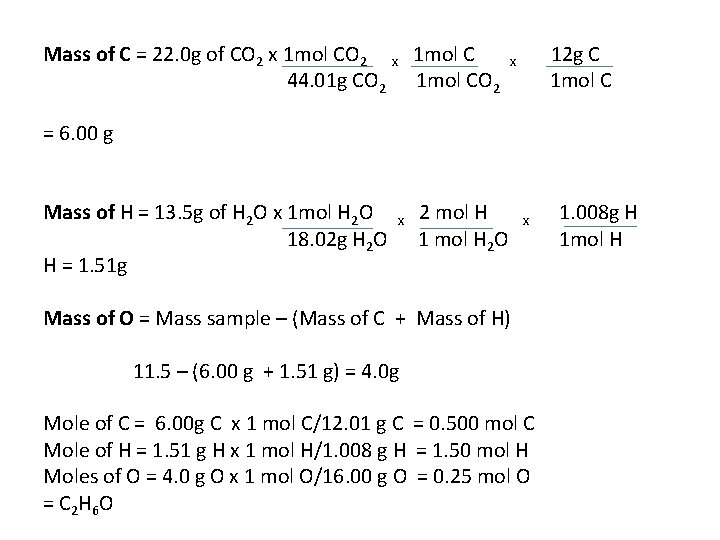
Mass of C = 22. 0 g of CO 2 x 1 mol C 44. 01 g CO 2 1 mol CO 2 12 g C 1 mol C x = 6. 00 g Mass of H = 13. 5 g of H 2 O x 1 mol H 2 O 18. 02 g H 2 O H = 1. 51 g x 2 mol H 1 mol H 2 O x Mass of O = Mass sample – (Mass of C + Mass of H) 11. 5 – (6. 00 g + 1. 51 g) = 4. 0 g Mole of C = 6. 00 g C x 1 mol C/12. 01 g C = 0. 500 mol C Mole of H = 1. 51 g H x 1 mol H/1. 008 g H = 1. 50 mol H Moles of O = 4. 0 g O x 1 mol O/16. 00 g O = 0. 25 mol O = C 2 H 6 O 1. 008 g H 1 mol H

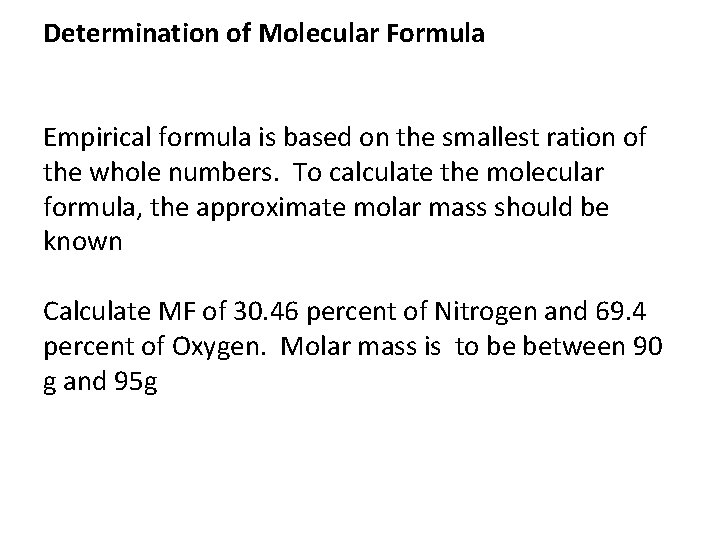
Determination of Molecular Formula Empirical formula is based on the smallest ration of the whole numbers. To calculate the molecular formula, the approximate molar mass should be known Calculate MF of 30. 46 percent of Nitrogen and 69. 4 percent of Oxygen. Molar mass is to be between 90 g and 95 g

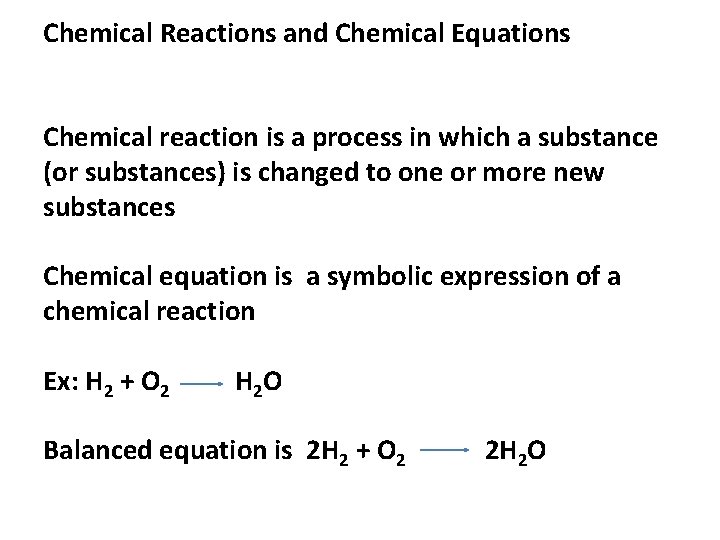
Chemical Reactions and Chemical Equations Chemical reaction is a process in which a substance (or substances) is changed to one or more new substances Chemical equation is a symbolic expression of a chemical reaction Ex: H 2 + O 2 H 2 O Balanced equation is 2 H 2 + O 2 2 H 2 O

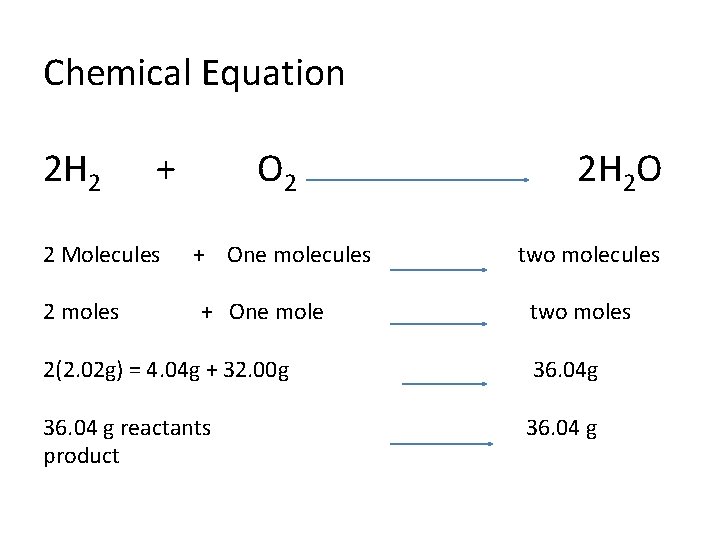
Chemical Equation 2 H 2 + 2 Molecules 2 moles O 2 + One molecules + One mole 2 H 2 O two molecules two moles 2(2. 02 g) = 4. 04 g + 32. 00 g 36. 04 g reactants product 36. 04 g

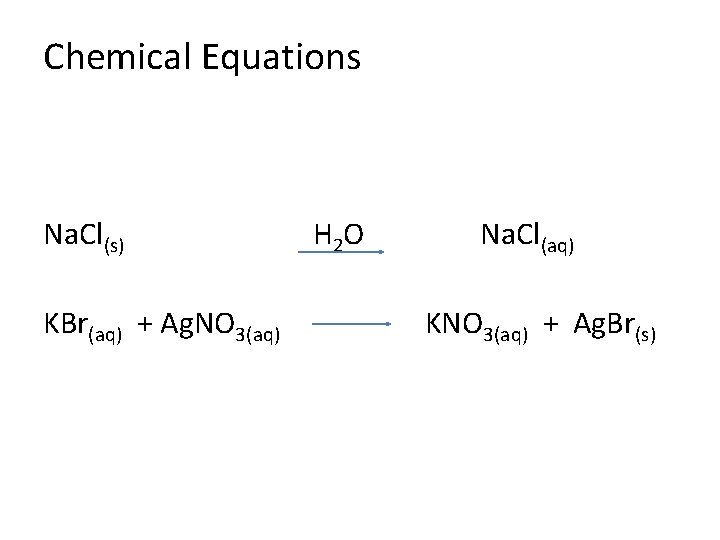
Chemical Equations Na. Cl(s) KBr(aq) + Ag. NO 3(aq) H 2 O Na. Cl(aq) KNO 3(aq) + Ag. Br(s)

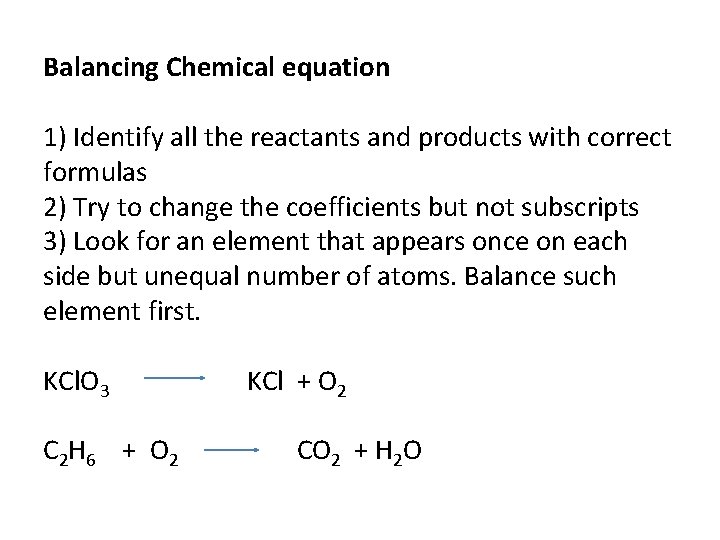
Balancing Chemical equation 1) Identify all the reactants and products with correct formulas 2) Try to change the coefficients but not subscripts 3) Look for an element that appears once on each side but unequal number of atoms. Balance such element first. KCl. O 3 C 2 H 6 + O 2 KCl + O 2 CO 2 + H 2 O

Stoichiometry of the reaction Stoichiometry is the quantitative study of the reactants and products in a chemical reaction

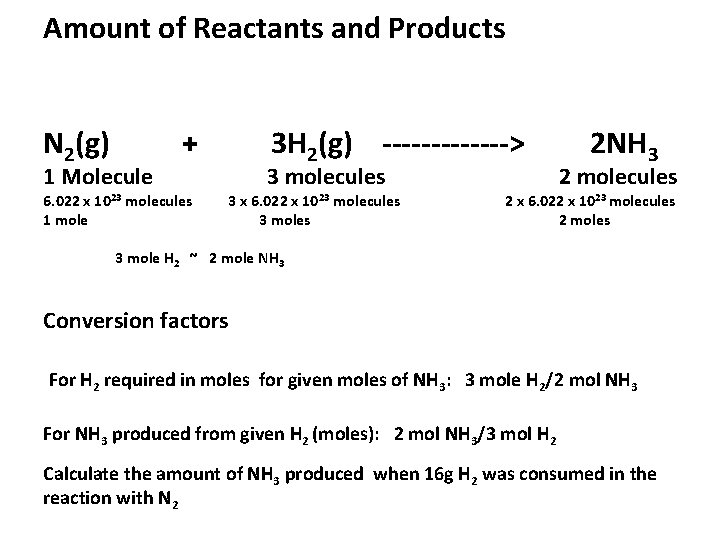
Amount of Reactants and Products N 2(g) 1 Molecule + 6. 022 x 1023 molecules 1 mole 3 H 2(g) -------> 3 molecules 3 x 6. 022 x 1023 molecules 3 moles 2 NH 3 2 molecules 2 x 6. 022 x 1023 molecules 2 moles 3 mole H 2 ~ 2 mole NH 3 Conversion factors For H 2 required in moles for given moles of NH 3: 3 mole H 2/2 mol NH 3 For NH 3 produced from given H 2 (moles): 2 mol NH 3/3 mol H 2 Calculate the amount of NH 3 produced when 16 g H 2 was consumed in the reaction with N 2

Summary of Stoichiometry 1) Write the balanced equation for the reaction 2) Convert the given amount of the reactant into moles 3) Use the mole ratio from the balanced equation to calculate the number of moles of product formed 4) Convert the moles of product into grams

Limiting reagents and excess reagents 1) The reagent used up first in the reaction is called Limiting reagent 2) The reactant present in quantities greater than necessary to react with the quantity of limiting reagent is called excess reagent Determine the limiting reagent when 4 mols CO and 6 mols H 2 used to produce methanol CO (g) + 2 H 2 (g) CH 3 OH (g)

Reaction yield Based on balanced equation, the yield of product can be determined. This is called theoretical yield The actual yield is the amount of the product actually obtained from a reaction %yield = actual yield/theoretical yield x 100