Chemistry Grade 10 Advanced Unit 1 STRUCTURE AND














- Slides: 35

Chemistry Grade 10 Advanced Unit 1 STRUCTURE AND BONDING IN MATTER Name: ------------------- Class: ------ 1


Structure and properties ﻭﺍﻟﺨﺼﺎﺋﺺ ﺍﻟﺘﺮﻛﻴﺐ 17. 1 Describe the distribution of mass and charge within an atom and deduce the numbers of protons, neutrons and electrons present in both atoms and ions, given proton and nucleon numbers. ﻓﻲ ﺍﻟﻤﻮﺟﻮﺩﺓ ﻭﺍﻻﻟﻜﺘﺮﻭﻧﺎﺕ ﻭﺍﻟﻨﻴﺘﺮﻭﻧﺎﺕ ﺍﻟﺒﺮﻭﺗﻮﻧﺎﺕ ﻋﺪﺩ ﻣﻨﻬﺎ ﻭﻳﺴﺘﻨﺘﺞ ﺫﺭﺓ ﺿﻤﻦ ﺍﻟﻜﻬﺮﺑﺎﺋﻴﺔ ﻭﺍﻟﺸﺤﻨﺔ ﺍﻟﻜﺘﻠﺔ ﺗﻮﺯﻳﻊ ﻳﺼﻒ 17. 1. ﻭﺍﻟﻨﻴﻜﻠﻴﻮﻧﺎﺕ ﺍﻟﺒﺮﻭﺗﻮﻧﺎﺕ ﻋﺪﺩ ﻋﻄﻰ ﻋﻨﺪﻣﺎ ، ﺍﻷﻴﻮﻧﺎﺕ ﻭﻓﻲ ﺍﻟﺬﺭﺍﺕ Objectives Students will be able to: 1. Understand that atoms are made up of a nucleus consisting of protons and neutrons surrounded by electrons in specific orbital's or shells. 2. Define and use the terms proton number, Nucleon number. 3. Describe the distribution of mass and charge within an atom and Deduce the numbers of protons, neutrons and electrons present in both atoms and ions, given proton and nucleon numbers. Key Vocabulary Atom Nucleus Proton Neutron Electron Orbital’s – Shells Ions Proton number Nucleon number Atoms are made up of three different subatomic particles: protons (p), neutrons (n) and electrons (e). The nucleus is at the centre of the atom, and contains the protons and neutrons and the electrons in specific orbital's around the nucleus. Atomic Number “Proton Number” (Z): Number of protons. Mass Number “Nucleon Number” (A): Number of protons + Number of neutrons. http: //www. yo utube. com/wa tch? v=Gsf 7 jh Wr 6 ng http: //www. yo utube. com/wa tch? v=EP 9 Et P 3 g. RZo&feat ure=related * New Chemistry for you : 3034 3


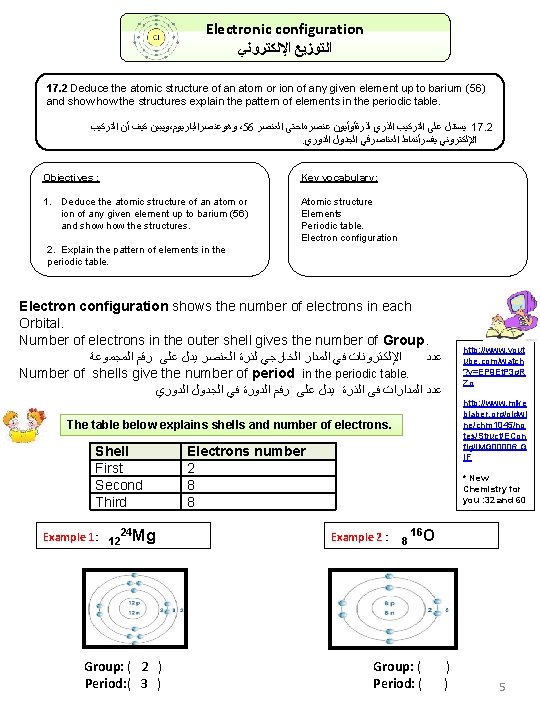
Electronic configuration ﺍﻟﺘﻮﺯﻳﻊ ﺍﻹﻟﻜﺘﺮﻭﻧﻲ 17. 2 Deduce the atomic structure of an atom or ion of any given element up to barium (56) and show the structures explain the pattern of elements in the periodic table. ﺍﻟﺘﺮﻛﻴﺐ ﺃﻦ ﻛﻴﻒ ﻭﻳﺒﻴﻦ ، ﻭﻫﻮﻋﻨﺼﺮﺍﻟﺒﺎﺭﻳﻮﻡ ،56 ﺍﻟﻌﻨﺼﺮ ﻋﻨﺼﺮﻣﺎﺣﺘﻰ ﻟﺬﺭﺓﺄﻮﺃﻴﻮﻥ ﺍﻟﺬﺭﻱ ﺍﻟﺘﺮﻛﻴﺐ ﻋﻠﻰ ﻳﺴﺘﺪﻝ 17. 2. ﺍﻟﺪﻭﺭﻱ ﺍﻟﺠﺪﻭﻝ ﺍﻟﻌﻨﺎﺻﺮﻓﻲ ﻳﻔﺴﺮﺃﻨﻤﺎﻁ ﺍﻹﻟﻜﺘﺮﻭﻧﻲ Objectives : Key vocabulary: 1. Deduce the atomic structure of an atom or ion of any given element up to barium (56) and show the structures. Atomic structure Elements Periodic table. Electron configuration 2. Explain the pattern of elements in the periodic table. Electron configuration shows the number of electrons in each Orbital. Number of electrons in the outer shell gives the number of Group. ﺍﻟﻤﺠﻤﻮﻋﺔ ﺭﻗﻢ ﻋﻠﻰ ﻳﺪﻝ ﺍﻟﻌﻨﺼﺮ ﻟﺬﺭﺓ ﺍﻟﺨﺎﺭﺟﻲ ﺍﻟﻤﺪﺍﺭ ﻓﻲ ﺍﻹﻟﻜﺘﺮﻭﻧﺎﺕ ﻋﺪﺩ Number of shells give the number of period in the periodic table. ﺍﻟﺪﻭﺭﻱ ﺍﻟﺠﺪﻭﻝ ﻓﻲ ﺍﻟﺪﻭﺭﺓ ﺭﻓﻢ ﻋﻠﻰ ﻳﺪﻝ ﺍﻟﺬﺭﺓ ﻓﻰ ﺍﻟﻤﺪﺍﺭﺍﺕ ﻋﺪﺩ The table below explains shells and number of electrons. Shell First Second Third Example 1: 1224 Mg Group: ( 2 ) Period: ( 3 ) Electrons number 2 8 8 http: //www. yout ube. com/watch ? v=EP 9 Et. P 3 g. R Zo http: //www. mike blaber. org/oldwi ne/chm 1045/no tes/Struct/ECon fig/IMG 00006. G IF * New Chemistry for you : 32 and 60 Example 2 : 8 16 O Group: ( ) Period: ( ) 5

Exercises Q 1: Draw the electronic configuration of the following atoms and determine period and group number. : ﺍﻟﺪﻭﺭﺓ ﻭ ﺍﻟﻤﺠﻤﻮﻋﺔ ﺭﻗﻢ ﻭﺣﺪﺩ ﺍﻟﺠﺪﻭﻝ ﻓﻲ ﻟﻠﻌﻨﺎﺻﺮ ﺍﻻﻟﻜﺘﺮﻭﻧﻲ ﺍﻟﺘﻮﺯﻳﻊ ﺍﺭﺳﻢ Element Electronic Configuration Period Number Group Number 5 B 13 Al 9 F 19 K 2 He 6

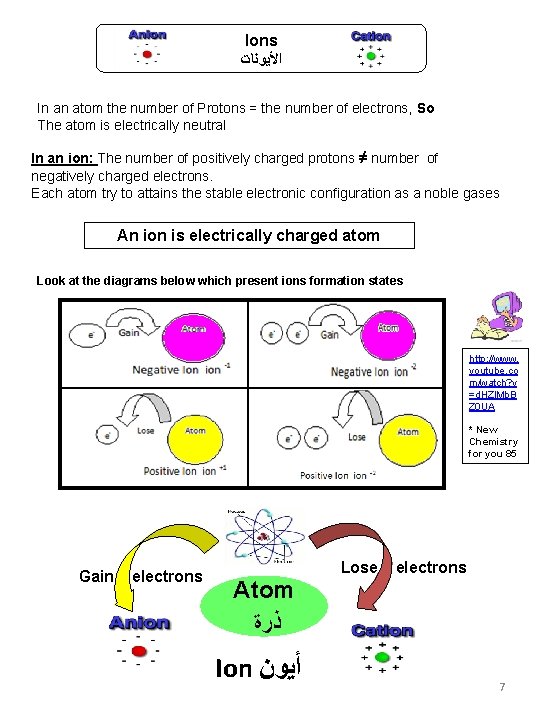
Ions ﺍﻷﻴﻮﻧﺎﺕ In an atom the number of Protons = the number of electrons, So The atom is electrically neutral In an ion: The number of positively charged protons ≠ number of negatively charged electrons. Each atom try to attains the stable electronic configuration as a noble gases An ion is electrically charged atom Look at the diagrams below which present ions formation states http: //www. youtube. co m/watch? v =d. HZl. Mb. B Z 0 UA * New Chemistry for you 85 Gain electrons Atom ﺫﺭﺓ Ion ﺃﻴﻮﻥ Lose electrons 7

Writing activity http: //www. you tube. com/watc h? v=Qqjc. Cvz Wwww&featur e=Play. List&p= C 9 D 5927352 B A 3 C 33&playne xt_from=PL&pl aynext=1&inde x=4 http: //www. you tube. com/watc h? v=x. Tx_DWb o. EVs&feature= related Use the above diagram to write a short story between sodium and chlorine. ---------------------------------------------------------------------------------------- Support stud ent’s English la nguage b y doing this activity. --------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 8

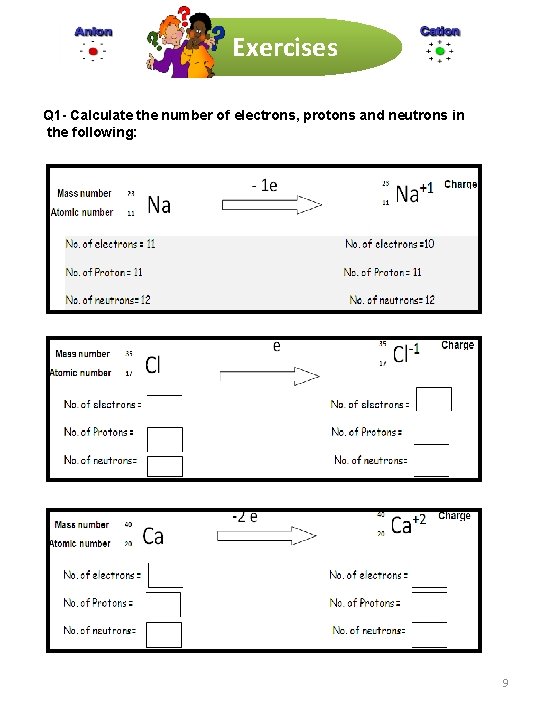
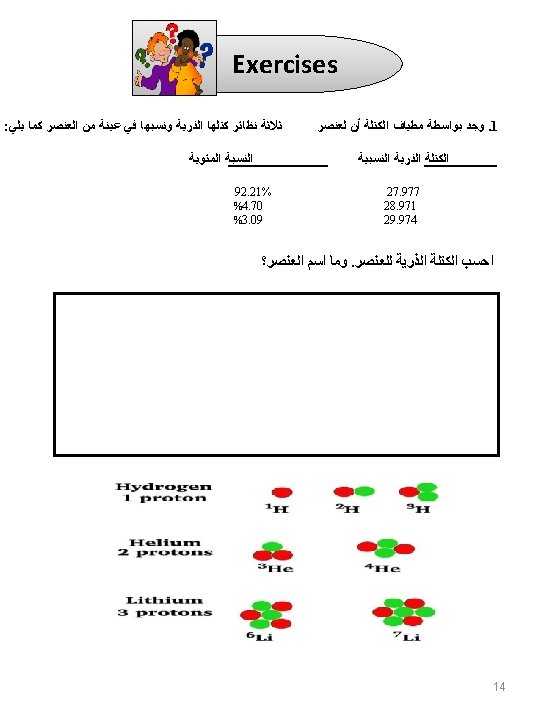
Exercises Q 1 - Calculate the number of electrons, protons and neutrons in the following: 9

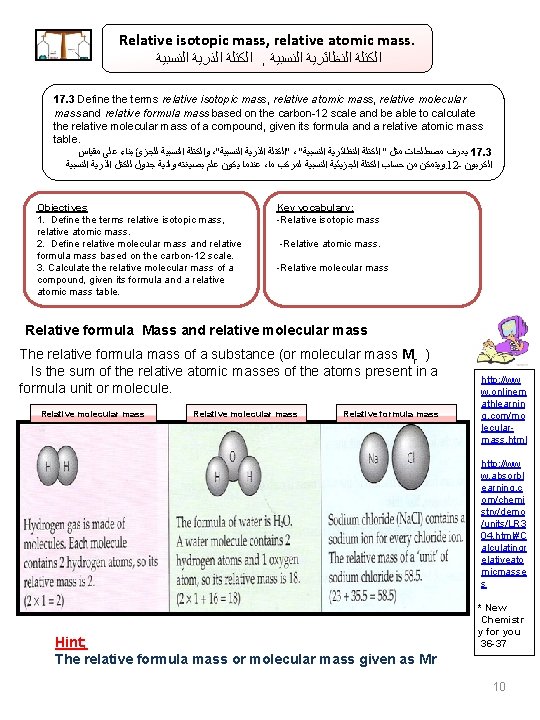
Relative isotopic mass, relative atomic mass. ﺍﻟﻨﺴﺒﻴﺔ ﺍﻟﺬﺭﻳﺔ ﺍﻟﻜﺘﻠﺔ , ﺍﻟﻨﺴﺒﻴﺔ ﺍﻟﻨﻈﺎﺋﺮﻳﺔ ﺍﻟﻜﺘﻠﺔ 17. 3 Define the terms relative isotopic mass, relative atomic mass, relative molecular mass and relative formula mass based on the carbon-12 scale and be able to calculate the relative molecular mass of a compound, given its formula and a relative atomic mass table. ﻣﻘﻴﺎﺱ ﻋﻠﻰ ﺑﻨﺎﺀ ﻟﻠﺠﺰﺉ ﺍﻟﻨﺴﺒﻴﺔ ﻭﺍﻟﻜﺘﻠﺔ ،“ ﺍﻟﻨﺴﺒﻴﺔ ﺍﻟﺬﺭﻳﺔ ”ﺍﻟﻜﺘﻠﺔ ، “ ﺍﻟﻨﺴﺒﻴﺔ ﺍﻟﻨﻈﺎﺋﺮﻳﺔ ﺍﻟﻜﺘﻠﺔ ” ﻣﺜﻞ ﻣﺼﻄﻠﺤﺎﺕ ﻳﻌﺮﻑ 17. 3 ﺍﻟﻨﺴﺒﻴﺔ ﺍﻟﺬﺭﻳﺔ ﻟﻠﻜﺘﻞ ﺟﺪﻭﻝ ﻭﻟﺪﻳﺔ ﺑﺼﻴﻐﺘﻪ ﻋﻠﻢ ﻳﻜﻮﻥ ﻋﻨﺪﻣﺎ ، ﻣﺎ ﻟﻤﺮﻛﺐ ﺍﻟﻨﺴﺒﻴﺔ ﺍﻟﺠﺰﻳﺌﻴﺔ ﺍﻟﻜﺘﻠﺔ ﺣﺴﺎﺏ ﻣﻦ ﻭﻳﺘﻤﻜﻦ. 12 - ﺍﻟﻜﺮﺑﻮﻥ Objectives 1. Define the terms relative isotopic mass, relative atomic mass. 2. Define relative molecular mass and relative formula mass based on the carbon-12 scale. 3. Calculate the relative molecular mass of a compound, given its formula and a relative atomic mass table. Key vocabulary: -Relative isotopic mass -Relative atomic mass. -Relative molecular mass Relative formula Mass and relative molecular mass The relative formula mass of a substance (or molecular mass Mr ) Is the sum of the relative atomic masses of the atoms present in a formula unit or molecule. Relative molecular mass Relative formula mass http: //ww w. onlinem athlearnin g. com/mo lecularmass. html http: //ww w. absorbl earning. c om/chemi stry/demo /units/LR 3 04. html#C alculatingr elativeato micmasse s Hint; The relative formula mass or molecular mass given as Mr * New Chemistr y for you 36 -37 10

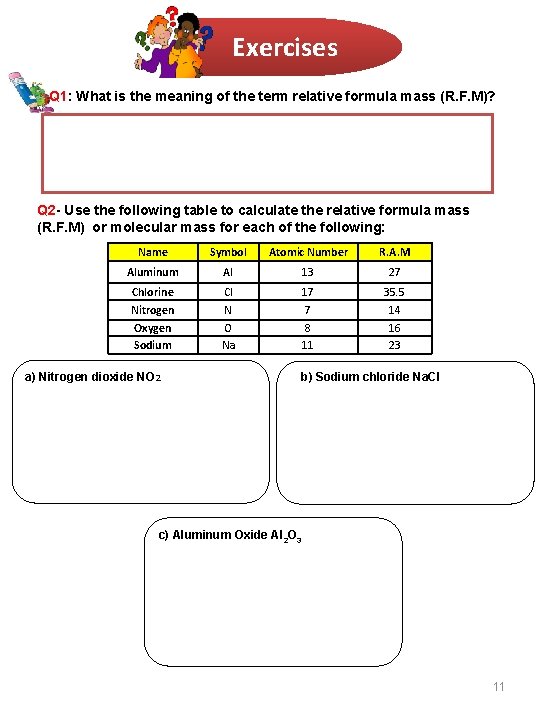
Exercises Q 1: What is the meaning of the term relative formula mass (R. F. M)? Q 2 - Use the following table to calculate the relative formula mass (R. F. M) or molecular mass for each of the following: Name Symbol Atomic Number R. A. M Aluminum Al 13 27 Chlorine Cl 17 35. 5 Nitrogen N 7 14 Oxygen Sodium O Na 8 11 16 23 a) Nitrogen dioxide NO 2 b) Sodium chloride Na. Cl c) Aluminum Oxide Al 2 O 3 11

Spectrometry and isotopes ﻭﺍﻟﻨﻈﺎﺋﺮ ﺍﻟﻄﻴﻒ ﻗﻴﺎﺱ 17. 4 Know that mass spectrometry can furnish information on relative isotopic masses and isotopic abundance. ﻭﺍﻟﻜﺜﺮﺓﺎﻟﻨﺴﺒﻴﺓﻠﻠﻨﻈﺎﺋﺮ ﺍﻟﻨﺴﺒﻴﺔ ﺍﻟﻨﻈﺎﺋﺮیﺔ ﺍﻟﻜﺘﻞ ﻋﻦ یﻮﻓﺮﻣﻌﻠﻮﻣﺎﺕ ﺃﻦ یﻤﻜﻦ ﺍﻟﻜﺘﻠﻲ ﺍﻟﻄﻴﻒ ﺗﺤﻠﻴﻞ ﺃﻦ یﻌﺮﻑ 17. 4 17. 5 Know that isotopes can be distinguished by their different numbers of neutrons and explain why the relative atomic mass of many elements is not a whole number. ﺍﻟﻜﺘﻠﺔ ﻟﻤﺎﺫﺍ ﻓﻴﻬﺎﻭیﺸﺮﺡ ﺍﻟﻤﻮﺟﻮﺩﺓ ﻟﻠﻨﻴﺘﺮﻭﻧﺎﺕ ﺍﻟﻤﺨﺘﻠﻔﺔ ﺍﻷﻌﺪﺍﺩ ﺧﻼﻝ ﺗﻤﻴﻴﺰﺍﻟﻨﻈﺎﺋﺮﻣﻦ ﺑﺎﻹﻣﻜﺎﻥ ﺃﻨﻪ یﻌﺮﻑ 17. 5 . ﺻﺤﻴﺤﺔ ﺃﻌﺪﺍﺩ ﻣﻦ ﺍﻟﻌﻨﺎﺻﺮﻏﻴﺮﻣﻜﻮﻧﺔ ﻣﻦ ﻟﻠﻌﺪیﺪ ﺍﻟﻨﺴﺒﻴﺔ ﺍﻟﺬﺭیﺔ Key Vocabulary Objectives: 1. Describe the mass spectrometer. Mass 2. Explain how mass spectrometry can give Spectrometer information about masses and abundance of Isotopic isotopes. Abundance 3. Relate the difference in isotopes to the different numbers of neutrons. 4. Explain why the relative atomic mass of many elements is not a whole number. ﻣﺨﻄﻄ ﺍﻟﺸﻜﻞ ﺑﻤﻄﻴﺎﻑ ﺍﻟﻜﺘﻠﺔ ﻭﻳﻤﺜﻞ ﺍﻟﺸﺤﻨﺔ ) ﺍﻷﻴﻮﻧﺎﺕ( ﻳﻌﺮﻑ ﻣﻮﺟﺒﺔ ﺍﻷﺠﺴﺎﻡ ﻛﺘﻞ ﻟﺪﺭﺍﺳﺔ ﺟﻬﺎﺯ ﻃﻮﺭ ﻟﻘﺪ ﻫﺬﻩ ﻣﻦ ﻟﻮﺍﺣﺪ. ﻟﻠﻨﻈﺎﺋﺮ ﺍﻟﻨﺴﺒﻴﺔ ﺍﻟﻜﺜﺮﺓ ﻭ ﻛﺘﻠﺘﻬﺎ ﺣﺴﺐ ﺍﻷﻴﻮﻧﺎﺕ ﻓﺼﻞ ﻓﻲ ﻭﺃﻬﻤﻴﺘﻪ ﺍﻷﺠﻬﺰﺓ A- ﺍﻟﻌﻴﻨﺔ ﺗﺒﺨﻴﺮ B- ﺍﻟﺘﺄﻴﻦ ﻏﺮﻓﺔ C- ﺍﻷﻴﻮﻧﺎﺕ ﺗﺴﺮﻳﻊ D- ﺍﻟﻤﻐﻨﺎﻃﻴﺴﻲ ﺍﻟﻤﺠﺎﻝ E- (ﺍﻟﻤﺮﺍﻗﺒﺔ monitor) F- ﺍﻟﺘﻔﺮﻳﻎ ﻣﻀﺨﺔ ﺇﻟﻰ The mass spectrometer: is a device that separate The mass spectrometer: particles based on their mass. The samples must move through the following steps 1 -vaporization- heat the substance to turn it into gas 2 - Ionization – to form positive ions. 3 - Acceleration – to move the ions fast by an electric field. 4 - Deflection - by magnetic field where ions are separated according to their mass. 5 -detection-where a monitor displays graph of different ions according to their mass and abundance audio visual resource. http: //www 2. c hemistry. msu. edu/faculty/re usch/Virt. Txt. J ml/Spectrpy/M ass. Spec/ma sspec 1. htm * New Chemistry for you: 364 -6 Let students watch a short animation / video clip to help them to appreciate how a mass spectrometer works. 12

Isotopes ﺍﻟﻨﻈﺎﺋﺮ http: //www. c olorado. edu/ physics/200 0/isotopes/in dex. html * New Chemistry for you: 35 Isotopes are atoms differ in mass because they differ in the number of neutrons. The relative atomic mass of many elements is not a whole number because of the presence of isotopes. The average relative atomic mass is the weighted average for all isotopes of a given element based upon their relative percent abundance. . ﺍﻟﻨﻴﺘﺮﻭﻧﺎﺕ ﺑﻌﺪﺩ ﺗﺨﺘﻠﻒ ﻷﻨﻬﺎ ﺍﻟﺬﺭﻳﺔ ﺍﻟﻜﺘﻠﺔ ﻓﻲ ﺗﺨﺘﻠﻒ ﺫﺭﺍﺕ ﻫﻲ ﺍﻟﻨﻈﺎﺋﺮ ﻫﻮ ﺍﻟﻨﺴﺒﻴﺔ ﺍﻟﺬﺭﻳﺔ ﺍﻟﻜﺘﻠﺔ ﻣﻌﺪﻝ ﻭ ﺍﻟﻌﻨﺎﺻﺮ ﻟﺘﻠﻚ ﻧﻈﺎﺋﺮ ﻭﺟﻮﺩ ﺑﺴﺒﺐ ﺍﻟﺤﻘﻴﻘﻲ ﺍﻟﻌﺪﺩ ﻟﻴﺴﺖ ﺍﻟﻌﻨﺎﺻﺮ ﻣﻦ ﻟﻌﺪﺩ ﺍﻟﻨﺴﺒﻴﺔ ﺍﻟﺬﺭﻳﺔ ﺍﻟﻜﺘﻠﺔ . ﻟﺘﻮﺍﺟﺪﻫﺎ ﺍﻟﻤﺌﻮﻳﺔ ﺍﻟﻨﺴﺒﺔ ﻋﻠﻰ ﺑﻨﺎﺀ ﺍﻟﻤﻜﻮﻧﺔ ﺍﻟﻨﻈﺎﺋﺮ ﻟﺠﻤﻴﻊ ﺍﻟﻨﺴﺒﻲ ﺍﻟﻮﺯﻥ Example Chlorine exist as two naturally occurring isotopes Cl-35 and Cl-37. if the Cl-35 has an abundance of 75. 76% and Cl-37 has abundance of 24. 24% , determine the relative atomic mass of chlorine. Solution: Ar = = 35. 48 13


Bonding ﺍﻟﺮﻭﺍﺑﻂ 17. 6 describe ionic and covalent bonding. . (ﻭﺍﻟﺮﺍﺑﻄﺓﺎﻟﺘﺴﺎﻫﻤﻴﺔ ﺍﻟﺘﻜﺎﻓﺆﺎﻟﻜﻬﺮﺑﺎﺋﻲ ﺍﺭﺗﺒﺎﻁ ) ﺍﻟﺮﺍﺑﻄﺓﺎﻷیﻮﻧﻴﺔ یﺼﻒ 17. 6 Objectives: 1. Relate types of bonding between atoms to its electron configuration. 2 - Show the role of valence electrons in determining the type of bonds between atoms. 3 - Represent ionic and covalent bonds using Lewis structures Key Vocabulary Electron configuration Valence electrons Ionic bonds Covalent bonds Lewis structures chemical bond ( ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ ) ﺍﻟﺮﺍﺑﻄﺔ is a force of attraction between the atoms which holds them together. The atoms combine with one another to reach a noble gas electronic configuration and become stable and this is why atoms banded together. http: //www. youtu be. com/watch? v =Qqjc. Cvz. Wwww Octet rule ( ﺍﻟﺜﻤﺎﻧﻴﺔ ﻗﺎﻋﺪﺓ ) When atoms combine to form a chemical bond, they gain, lose or share electrons in such a way that each atom gets noble gas configuration or 8 electrons in its outer most shell (or 2 electrons in the outer most K shell. ) http: //www. youtu be. com/watch? v =yjge 1 Wd. CFPs http: //www. youtu be. com/watch? v =WXy. FMJ 0 e. JA 0 &feature=related Lewis electron dot structure ( ﻟﻮﻳﺲ ﺗﺮﻛﻴﺐ ) shows the symbols of elements along with their valence electrons as dots around them. Valence (outer most) electrons are usually the only electrons used in chemical bonds. So, only valence electrons are shown in electron dot structure. As you are familiar that all of the group 1 elements have 1 valence electron so they are shown with 1 dot. Group 2 elements have 2 dots; group 3 elements have 3 dots and so on. • Li • • Be • • B • • • C • • • : N • • • : O • . . : F • . . : Ne : . . 15

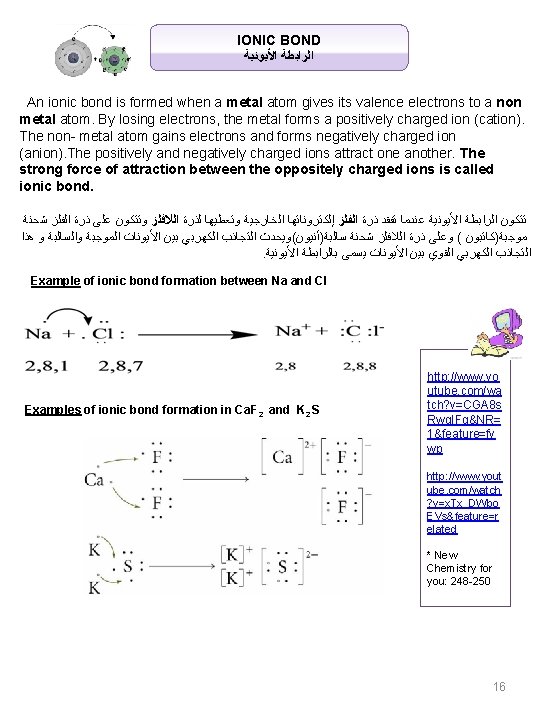
IONIC BOND ﺍﻷﻴﻮﻧﻴﺔ ﺍﻟﺮﺍﺑﻄﺔ An ionic bond is formed when a metal atom gives its valence electrons to a non metal atom. By losing electrons, the metal forms a positively charged ion (cation). The non- metal atom gains electrons and forms negatively charged ion (anion). The positively and negatively charged ions attract one another. The strong force of attraction between the oppositely charged ions is called ionic bond. ﺷﺤﻨﺔ ﺍﻟﻔﻠﺰ ﺫﺭﺓ ﻋﻠﻰ ﻭﺗﺘﻜﻮﻥ ﺍﻟﻼﻓﻠﺰ ﻟﺬﺭﺓ ﻭﺗﻌﻄﻴﻬﺎ ﺍﻟﺨﺎﺭﺟﻴﺔ ﺇﻟﻜﺘﺮﻭﻧﺎﺗﻬﺎ ﺍﻟﻔﻠﺰ ﺫﺭﺓ ﺗﻔﻘﺪ ﻋﻨﺪﻣﺎ ﺍﻷﻴﻮﻧﻴﺔ ﺍﻟﺮﺍﺑﻄﺔ ﺗﺘﻜﻮﻥ ﻫﺬﺍ ﻭ ﻭﺍﻟﺴﺎﻟﺒﺔ ﺍﻟﻤﻮﺟﺒﺔ ﺍﻷﻴﻮﻧﺎﺕ ﺑﻴﻦ ﺍﻟﻜﻬﺮﺑﻲ ﺍﻟﺘﺠﺎﺫﺏ ﺳﺎﻟﺒﺔ)ﺃﻨﻴﻮﻥ(ﻭﻳﺤﺪﺙ ﺷﺤﻨﺔ ﺍﻟﻼﻓﻠﺰ ﺫﺭﺓ ﻭﻋﻠﻰ ( ﻣﻮﺟﺒﺔ)ﻛﺎﺗﺒﻮﻥ . ﺍﻷﻴﻮﻧﻴﺔ ﺑﺎﻟﺮﺍﺑﻄﺔ ﻳﺴﻤﻰ ﺍﻷﻴﻮﻧﺎﺕ ﺑﻴﻦ ﺍﻟﻘﻮﻱ ﺍﻟﻜﻬﺮﺑﻲ ﺍﻟﺘﺠﺎﺫﺏ Example of ionic bond formation between Na and Cl Examples of ionic bond formation in Ca. F 2 and K 2 S http: //www. yo utube. com/wa tch? v=CGA 8 s Rwq. IFg&NR= 1&feature=fv wp http: //www. yout ube. com/watch ? v=x. Tx_DWbo EVs&feature=r elated * New Chemistry for you: 248 -250 16

Exercises Q 1: Try for ionic bond formation in the following elements: 1. Al and Br 2. Mg and N 3. Mg and O 17

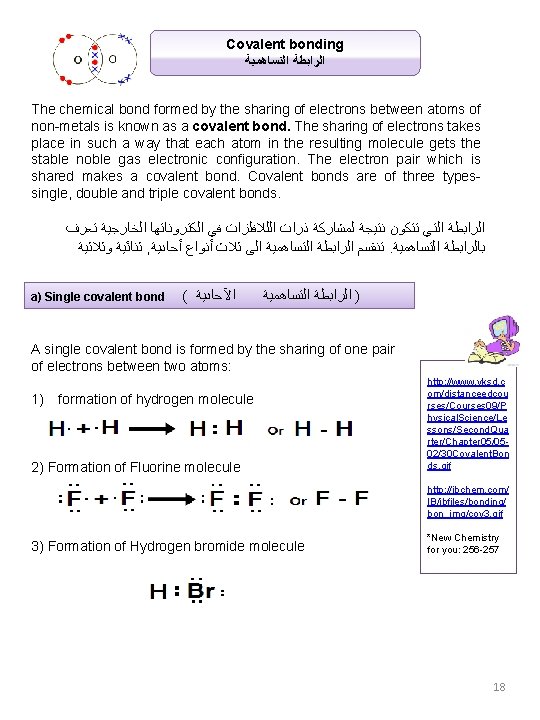
Covalent bonding ﺍﻟﺘﺴﺎﻫﻤﻴﺔ ﺍﻟﺮﺍﺑﻄﺔ The chemical bond formed by the sharing of electrons between atoms of non-metals is known as a covalent bond. The sharing of electrons takes place in such a way that each atom in the resulting molecule gets the stable noble gas electronic configuration. The electron pair which is shared makes a covalent bond. Covalent bonds are of three types- single, double and triple covalent bonds. ﺗﻌﺮﻑ ﺍﻟﺨﺎﺭﺟﻴﺔ ﺍﻟﻜﺘﺮﻭﻧﺎﺗﻬﺎ ﻓﻲ ﺍﻟﻠﻼﻓﻠﺰﺍﺕ ﺫﺭﺍﺕ ﻟﻤﺸﺎﺭﻛﺔ ﻧﺘﻴﺠﺔ ﺗﺘﻜﻮﻥ ﺍﻟﺘﻲ ﺍﻟﺮﺍﺑﻄﺔ ﻭﺛﻼﺛﻴﺔ ﺛﻨﺎﺋﻴﺔ , ﺃﺤﺎﺩﻳﺔ ﺃﻨﻮﺍﻉ ﺛﻼﺙ ﺍﻟﻰ ﺍﻟﺘﺴﺎﻫﻤﻴﺔ ﺍﻟﺮﺍﺑﻄﺔ ﺗﻨﻘﺴﻢ . ﺍﻟﺘﺴﺎﻫﻤﻴﺔ ﺑﺎﻟﺮﺍﺑﻄﺔ a) Single covalent bond ( ﺍﻵﺤﺎﺩﻳﺔ ﺍﻟﺘﺴﺎﻫﻤﻴﺔ ) ﺍﻟﺮﺍﺑﻄﺔ A single covalent bond is formed by the sharing of one pair of electrons between two atoms: 1) formation of hydrogen molecule 2) Formation of Fluorine molecule http: //www. yksd. c om/distanceedcou rses/Courses 09/P hysical. Science/Le ssons/Second. Qua rter/Chapter 05/0502/30 Covalent. Bon ds. gif http: //ibchem. com/ IB/ibfiles/bonding/ bon_img/cov 3. gif 3) Formation of Hydrogen bromide molecule *New Chemistry for you: 256 -257 18


Metallic bonding ﺍﻟﻔﻠﺰﻳﺔ ﺍﻟﺮﺍﺑﻄﺔ 17. 7 Explain metallic bonding in terms of a lattice of positive ions surrounded by a sea of mobile electrons and explain the physical properties of metals and alloys in terms of this bonding. ﺍﻟﻔﻴﺰﻳﺎﺋﻴﺔ ﻭﻳﻔﺴﺮﺍﻟﺨﺼﺎﺋﺺ ، ﺍﻟﻤﺘﺤﺮﻛﺔ ﺍﻹﻟﻜﺘﺮﻭﻧﺎﺕ ﻣﻦ ﺑﺴﺤﺎﺑﺔ ﺍﻟﻤﺤﺎﻃﺔ ﺍﻟﻤﻮﺟﺒﺔ ﺍﻷﻴﻮﻧﺎﺕ ﻣﻦ ﺷﺒﻜﺔ ﺑﺪﻻﻟﺔ ﺍﻟﻔﻠﺰﻳﺔ ﺍﻟﺮﺍﺑﻄﺔ ﻳﻔﺴﺮ 17. 7 ﺍﻷﺮﺗﺒﺎﻁ ﻫﺬﺍ ﺑﺪﻻﻟﺔ ﻭﺍﻟﺴﺒﺎﺋﻚ ﻟﻠﻔﻠﺰﺍﺕ Objectives: 1. Explain metallic bonding. 2. Explain the physical properties of alloys. 3. Write an example of alloy. Key vocabulary: metallic bonding drift Delocalized Alloy Each of magnesium atom gives up its electrons from its outer shell into the “sea” Or “ cloud” of electrons. The electrons can drift about in the metal. We call them ‘delocalized ’ electrons. delocalized means "not fixed in one place" or "free to move". ﺃﻮ ﺑﺤﺮ ﺍﻟﻰ ﻭﺗﺘﺤﻮﻝ ﺍﻟﺨﺎﺭﺟﻲ ﻏﻼﻓﻬﺎ ﻣﻦ ﺍﻟﻜﺘﺮﻭﻧﺎﺕ ﺗﻔﻘﺪ ﺍﻟﻤﻐﻨﻴﺴﻴﻮﻡ ﻣﻦ ﺫﺭﺓ ﻛﻞ . ﻣﺘﻤﺮﻛﺰﺓ ﻏﻴﺮ ﺍﻟﻜﺘﺮﻭﻧﺎﺕ ﻧﺴﻤﻴﻬﺎ . ﺍﻟﺬﺭﺓ ﺣﻮﻝ ﺍﻻﻟﻜﺘﺮﻭﻧﺎﺕ ﻣﻦ " "ﺳﺤﺎﺑﺔ ﺍﻟﺘﺤﺮﻙ ﺣﺮﻳﺔ ﻟﻬﺎ ” ﺃﻮ " ﻭﺍﺣﺪ ﻣﻜﺎﻥ ﻓﻲ ﺛﺎﺑﺘﺔ "ﻟﻴﺴﺖ ﺗﻌﻨﻲ ﻣﺘﻤﺮﻛﺰﺓ ﻏﻴﺮ ﺍﻟﻜﺘﺮﻭﻧﺎﺕ http: //www. au setute. com. au /metallic. html. *New Chemistry for you: 268 20

Exercises Q 1: Mention the properties of metals: 1) 2) 3) 4) 5) 6) Q 2: Why metals are good conductors of electricity? ………………………………………………………. …. . …………………………………………………. … Q 3 - Compare between ionic and metallic bonding: 21

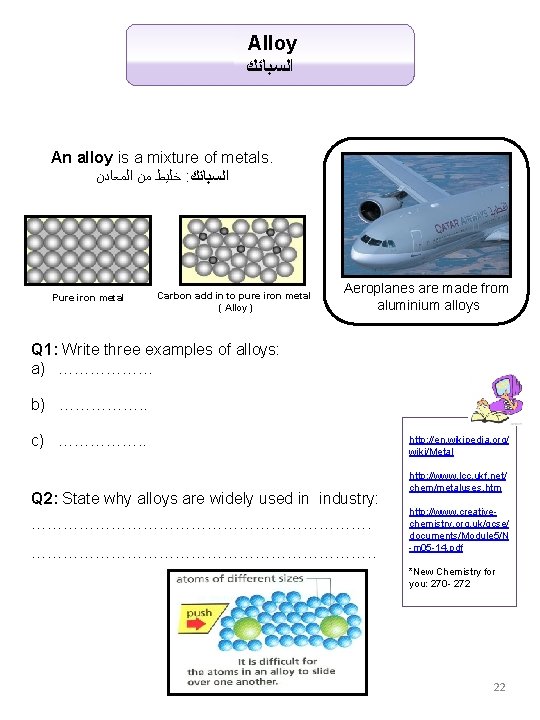
Alloy ﺍﻟﺴﺒﺎﺋﻚ An alloy is a mixture of metals. ﺍﻟﻤﻌﺎﺩﻥ ﻣﻦ ﺧﻠﻴﻂ : ﺍﻟﺴﺒﺎﺋﻚ Pure iron metal Carbon add in to pure iron metal ( Alloy ) Aeroplanes are made from aluminium alloys Q 1: Write three examples of alloys: a) ……………… b) ……………. . c) ……………. . Q 2: State why alloys are widely used in industry: ………………………………………………………. . http: //en. wikipedia. org/ wiki/Metal http: //www. lcc. ukf. net/ chem/metaluses. htm http: //www. creativechemistry. org. uk/gcse/ documents/Module 5/N -m 05 -14. pdf *New Chemistry for you: 270 - 272 22

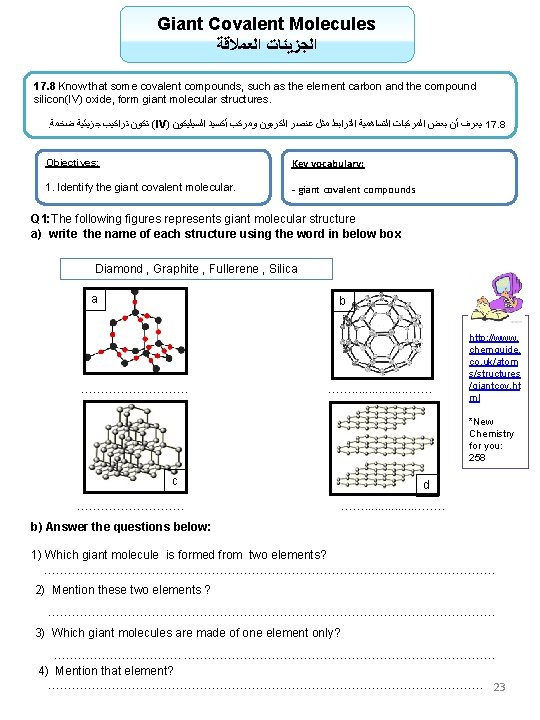
Giant Covalent Molecules ﺍﻟﻌﻤﻼﻗﺔ ﺍﻟﺠﺰﻳﺌﺎﺕ 17. 8 Know that some covalent compounds, such as the element carbon and the compound silicon(IV) oxide, form giant molecular structures. . ﺿﺨﻤﺔ ﺟﺰﻳﺌﻴﺔ ﺗﺮﺍﻛﻴﺐ ﺗﻜﻮﻥ (IV) ﺍﻟﺴﻴﻠﻴﻜﻮﻥ ﺃﻜﺴﻴﺪ ﻭﻣﺮﻛﺐ ﺍﻟﻜﺮﺑﻮﻥ ﻋﻨﺼﺮ ﻣﺜﻞ ﺍﻟﺘﺮﺍﺑﻂ ﺍﻟﺘﺴﺎﻫﻤﻴﺔ ﺍﻟﻤﺮﻛﺒﺎﺕ ﺑﻌﺾ ﺃﻦ ﻳﻌﺮﻑ 17. 8 Objectives: Key vocabulary: 1. Identify the giant covalent molecular. - giant covalent compounds Q 1: The following figures represents giant molecular structure a) write the name of each structure using the word in below box Diamond , Graphite , Fullerene , Silica a b …………… ……. . . . ……. http: //www. chemguide. co. uk/atom s/structures /giantcov. ht ml *New Chemistry for you: 258 c d …………… ……. . . . ……. b) Answer the questions below: 1) Which giant molecule is formed from two elements? …………………………………………………. . 2) Mention these two elements ? …………………………………………………. 3) Which giant molecules are made of one element only? ……………. . ………………………………… 4) Mention that element? ………………………………………………. 23

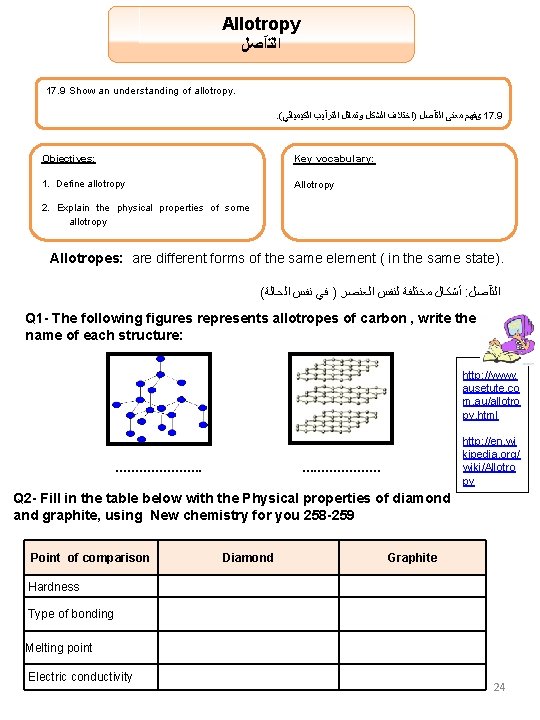
Allotropy ﺍﻟﺘآﺼﻞ 17. 9 Show an understanding of allotropy. . ( ﺍﻟﻜﻴﻤﻴﺎﺋﻲ ﺍﻟﺘﺮآﻴﺐ ﻭﺗﻤﺎﺛﻞ ﺍﻟﺸﻜﻞ )ﺍﺧﺘﻼﻑ ﺍﻟﺘآﺼﻞ ﻣﻌﻨﻰ یﻔﻬﻢ 17. 9 Objectives: Key vocabulary: 1. Define allotropy Allotropy 2. Explain the physical properties of some allotropy Allotropes: are different forms of the same element ( in the same state). ( ﺍﻟﺤﺎﻟﺔ ﻧﻔﺲ ﻓﻲ ) ﺍﻟﻌﻨﺼﺮ ﻟﻨﻔﺲ ﻣﺨﺘﻠﻔﺔ ﺃﺸﻜﺎﻝ : ﺍﻟﺘآﺼﻞ Q 1 - The following figures represents allotropes of carbon , write the name of each structure: http: //www. ausetute. co m. au/allotro py. html …………………. http: //en. wi kipedia. org/ wiki/Allotro py …. . …………… Q 2 - Fill in the table below with the Physical properties of diamond and graphite, using New chemistry for you 258 -259 Point of comparison Diamond Graphite Hardness Type of bonding Melting point Electric conductivity 24

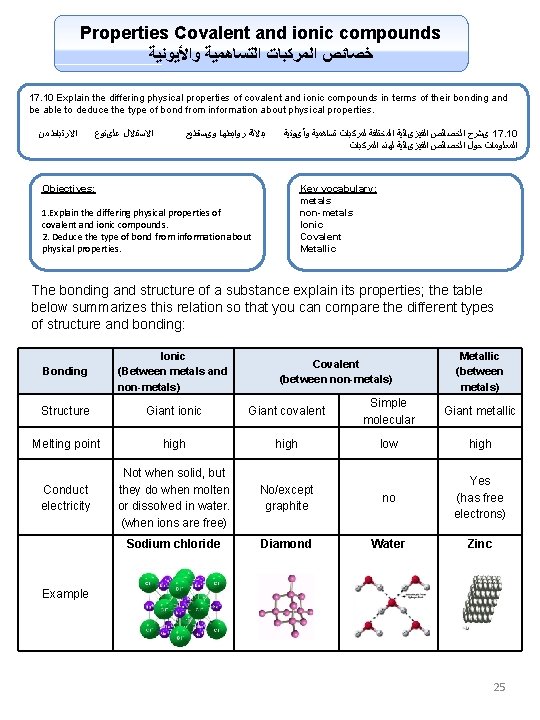
Properties Covalent and ionic compounds ﻭﺍﻷﻴﻮﻧﻴﺔ ﺍﻟﺘﺴﺎﻫﻤﻴﺔ ﺍﻟﻤﺮﻛﺒﺎﺕ ﺧﺼﺎﺋﺺ 17. 10 Explain the differing physical properties of covalent and ionic compounds in terms of their bonding and be able to deduce the type of bond from information about physical properties. ﻣﻦ ﺍﻻﺭﺗﺒﺎﻁ ﻋﻠىﻨﻮﻉ ﺍﻻﺳﺘﺪﻻﻝ ﻭیﺴﺘﻄﻴﻊ ﺭﻭﺍﺑﻄﻬﺎ ﺑﺪﻻﻟﺔ ﻭﺃیﻮﻧﻴﺔ ﺗﺴﺎﻫﻤﻴﺔ ﻟﻤﺮﻛﺒﺎﺕ ﺍﻟﻤﺨﺘﻠﻔﺔ ﺍﻟﻔﻴﺰیﺎﺋﻴﺔ ﺍﻟﺨﺼﺎﺋﺺ یﺸﺮﺡ 17. 10 ﺍﻟﻤﺮﻛﺒﺎﺕ ﻟﻬﺬﻩ ﺍﻟﻔﻴﺰیﺎﺋﻴﺔ ﺍﻟﺨﺼﺎﺋﺺ ﺣﻮﻝ ﺍﻟﻤﻌﻠﻮﻣﺎﺕ Objectives: Key vocabulary: metals non-metals Ionic Covalent Metallic 1. Explain the differing physical properties of covalent and ionic compounds. 2. Deduce the type of bond from information about physical properties. The bonding and structure of a substance explain its properties; the table below summarizes this relation so that you can compare the different types of structure and bonding: Bonding Ionic (Between metals and non-metals) Structure Giant ionic Giant covalent Simple molecular Giant metallic Melting point high low high Conduct electricity Not when solid, but they do when molten or dissolved in water. (when ions are free) No/except graphite no Yes (has free electrons) Sodium chloride Diamond Water Zinc Covalent (between non-metals) Metallic (between metals) Example 25

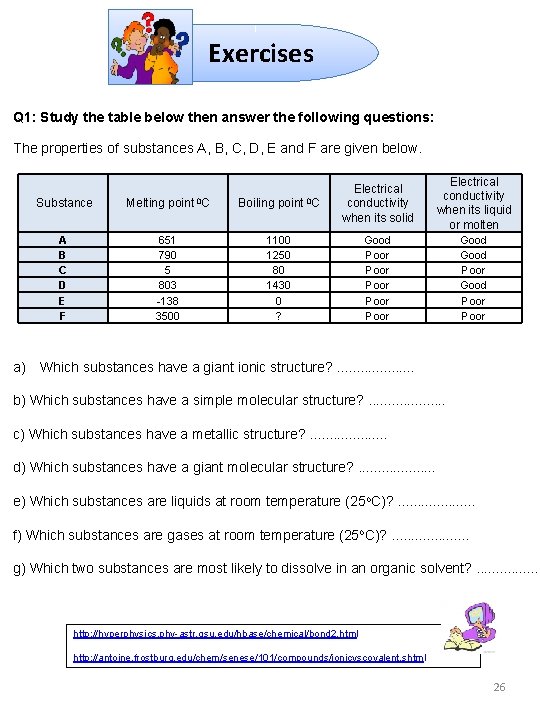
Exercises Q 1: Study the table below then answer the following questions: The properties of substances A, B, C, D, E and F are given below. Substance Melting point 0 C Boiling point 0 C Electrical conductivity when its solid A B C D E F 651 790 5 803 -138 3500 1100 1250 80 1430 0 ? Good Poor Poor Electrical conductivity when its liquid or molten Good Poor a) Which substances have a giant ionic structure? . . b) Which substances have a simple molecular structure? . . c) Which substances have a metallic structure? . . d) Which substances have a giant molecular structure? . . e) Which substances are liquids at room temperature (25 o. C)? . . f) Which substances are gases at room temperature (25 o. C)? . . g) Which two substances are most likely to dissolve in an organic solvent? . . . . http: //hyperphysics. phy-astr. gsu. edu/hbase/chemical/bond 2. html http: //antoine. frostburg. edu/chem/senese/101/compounds/ionicvscovalent. shtml 26

Ionic compounds ﺍﻷﻴﻮﻧﻴﺔ ﺍﻟﻤﺮﻛﺒﺎﺕ 17. 11 Explain why molten ionic compounds and solutions of ionic compounds conduct electricity. . ﺍﻟﻜﻬﺮﺑﺎﺀ ﺗﻮﺻﻞ ﺍﻷیﻮﻧﻴﺔ ﺍﻟﻤﺮﻛﺒﺎﺕ ﻭﻣﺤﺎﻟﻴﻞ ﺍﻟﻤﻨﺼﻬﺮﺓ ﺍﻷیﻮﻧﻴﺔ Objectives : Key vocabulary: 1. Molten ionic compounds conduct electricity. -Molten ionic compounds ﻟﻤﺎﺫﺍﺍﻟﻤﺮﻛﺒﺎﺕ یﺸﺮﺡ 17. 11 2. Solutions of ionic compounds conduct electricity. 3. Solid ionic compounds can’t conduct electricity. Ionic compounds made up of ions (charged particles). Solid sodium chloride contain fixed ions can’t move to the electrodes. So, it can’t conduct electricity. However, when they are melted or dissolved in water, the ions become free to move around. So, it can conduct electricity. Writing activity Write another paragraph for sodium chloride ---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 27

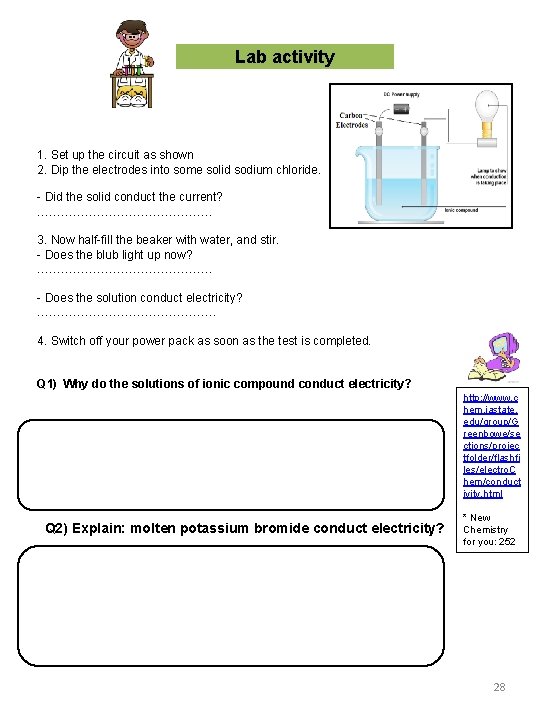
Lab activity 1. Set up the circuit as shown 2. Dip the electrodes into some solid sodium chloride. - Did the solid conduct the current? …………………. . 3. Now half-fill the beaker with water, and stir. - Does the blub light up now? …………………. . - Does the solution conduct electricity? …………………… 4. Switch off your power pack as soon as the test is completed. Q 1) Why do the solutions of ionic compound conduct electricity? http: //www. c hem. iastate. edu/group/G reenbowe/se ctions/projec tfolder/flashfi les/electro. C hem/conduct ivity. html Q 2) Explain: molten potassium bromide conduct electricity? * New Chemistry for you: 252 28

Writing chemical equations ﻛﺘﺎﺑﺔ ﺍﻟﻤﻌﺎﺩﻻﺕ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ 17. 12 Write equations with state symbols for simple reactions, including ionic equations for reactions in aqueous solution, given the formulae of reactants and products. ﻣﻌﺎﺩﻻﺕ ﻓﻴﻬﺎ ﺑﻤﺎ ، ﺑﺴﻴﻄﺔ ﻟﺘﻔﺎﻋﻼﺕ ﺍﻟﻤﺎﺩﺓ ﺣﺎﻟﺔ ﻋﻠﻰ ﺗﺪﻝ ﺍﻟﺮﻣﻮﺯﺍﻟﺘﻲ ﻋﻠﻰ ﺗﺤﺘﻮﻱ ﻣﻌﺎﺩﻻﺕ یﻜﺘﺐ 17. 12. ﻭﻧﻮﺍﺗﺠﻬﺎ ﺍﻟﻤﺘﻔﺎﻋﻠﺔ ﺍﻟﻤﻮﺍﺩ ﺻﻍ یﻌﺮﻑ ﻋﻨﺪﻣﺎ ، ﻣﺎﺋﻲ ﻣﺤﻠﻮﻝ ﻓﻲ ﺗﺤﺼﻞ ﻟﺘﻔﺎﻋﻼﺕ ﺃیﻮﻧﻴﺔ Objectives : Key vocabulary: Write equations with state symbols for simple reactions - symbols - reactions - ionic equations - aqueous solution -Reactants - products What is a chemical equation? ﻣﺎﻫﻲ ﺍﻟﻤﻌﺎﺩﻟﺔ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ ؟ When a chemical reaction occurs, it can be described by an equation. This shows the chemicals that react (called the reactants) on the left-hand side, and the chemicals that they produce (called the products) on the right-hand side. Unlike mathematical equations, the two sides are separated by an arrow, that indicates that the reactants form the products and not the other way round. How to write the chemical equations? ﻛﻴﻒ ﺗﻜﺘﺐ ﺍﻟﻤﻌﺎﺩﻟﺔ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ ﺍﻟﻜﻴﻤﻴﺎﺋﻲ ﺍﻟﺮﻣﺰ ﻟﻜﺘﺎﺑﺔ ﺍﻟﻼﺯﻣﺔ ﺑﺎﻟﺸﺮﻭﻁ ﺍﻟﺘﻘﻴﺪ ﻳﺠﺐ ﺍﻟﺮﻣﺰﻳﺔ ﺍﻟﻤﻌﺎﺩﻟﺔ ﻭﻟﻜﺘﺎﺑﺔ ﺭﻣﺰﻳﺔ ﺃﻮ ﻟﻔﻈﻴﺔ ﺗﻜﻮﻥ ﻗﺪ ﺍﻟﻤﻌﺎﺩﻟﺔ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ ﺑﺼﻴﻐﻬﺎ ﻓﺘﻜﺘﺐ ﺍﻟﻤﺮﻛﺒﺎﺕ ﺍﻣﺎ . H 2 , N 2, O 2 ﺛﻨﺎﺋﻴﺔ ﺟﺰﻳﺌﺎﺕ ﺷﻜﻞ ﻓﻲ ﺗﻜﺘﺐ ﺍﻟﻐﺎﺯﻳﺔ ﺍﻟﻌﻨﺎﺻﺮ , ﻟﻠﻤﺎﺩﺓ ﺗﺘﻢ ﺫﻟﻚ ﺑﻌﺪ . Mg, Na, C ﺍﻟﻤﻔﺮﺩﺓ ﺫﺭﺍﺗﻬﺎ ﺗﻜﺘﺐ ﻭﺍﻟﻜﺮﺑﻮﻥ ﺍﻟﻔﻠﺰﺍﺕ ﻣﺜﻞ ﺍﻟﺼﻠﺒﺔ ﺍﻟﻌﻨﺎﺻﺮ . Mg. O, Na. Cl. ﺍﻟﻨﺎﺗﺠﺔ ﺍﻟﺬﺭﺍﺕ ﻋﺪﺩ ﺗﺴﺎﻭﻱ ﺍﻟﻤﺘﻔﺎﻋﻠﺔ ﺍﻟﺬﺭﺍﺕ ﻋﺪﺩ ﺗﻜﻮﻥ ﺑﺤﻴﺚ ﺍﻟﻤﻌﺎﺩﻟﺔ ﻣﻮﺍﺯﻧﺔ Chemicals can be represented by their names or by their chemical symbols. Symbols of element gases are diatomic like H 2 , N 2, O 2. Solid elements can be represented by simples like Mg, Na, C, Al. Compounds are represented by their chemical formula like Mg. O, Na. Cl. For metals with different oxidation states , add Roman number when represented by their names e. g Copper(II) oxide (Cu. O)There are reversible reactions, which means that the reactants react together to form the products, but as soon as the products are formed, they start to react together to reform the reactants! Reversible reactions are indicated with a double arrow as shown in the example below: 29

ﺍﻟﻤﺎﺩﺓ ﺣﺎﻻﺕ ﻋﻦ ﺗﻌﺒﺮ ﺻﻐﻴﺮﺓ ﺭﻣﻮﺯ ﺗﻜﺘﺐ ﻭﺿﻮﺣﺎ ﺃﻜﺜﺮ ﺍﻟﻤﻌﺎﺩﻟﺔ ﻟﺘﻜﻮﻥ To make a chemical equation complete, the state of matter of each substance should also be included. This indicates whether the substance is: (s) (l) (g) (aq) solid liquid gas aqueous (dissolved in water) * In this example, solid magnesium ribbon burns in oxygen gas to form solid magnesium oxide: 2 Mg (s) + O 2 (g) -- 2 Mg. O (s) Student activity (1): Use your periodic table and table of poly atomic ions to write the chemical formulas for the following compounds. Show the following steps; Step one - Write the symbols for the elements or the polyatomic ions in the compound. Step two - Look up the valence numbers of the elements or the polyatomic ions involved and write them as superscripts to the right of the elemental symbols. http: //www. yout ube. com/watch ? v=YDJFf. Z 5 Wq ZQ&feature=Pl ay. List&p=C 983 2 A 956597 F 2 C 3 &playnext=1&pl aynext_from=P L&index=65 *New Chemistry for you: 26 -28 Step three - Use the correct combination of ions (and the polyatomic ions) to produce a compound with a net charge of zero. Multiple ions are indicated with subscripts. Teacher will be provide periodic table to students with a table of polyatomic ions 30

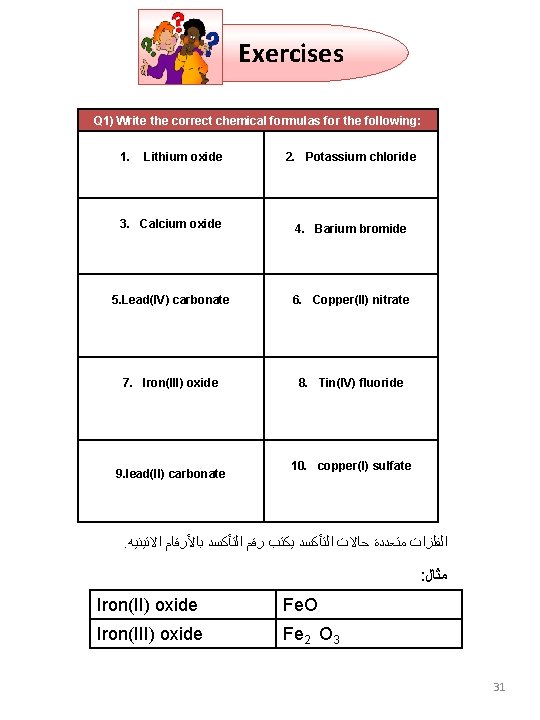
Exercises Q 1) Write the correct chemical formulas for the following: 1. Lithium oxide 2. Potassium chloride 3. Calcium oxide 4. Barium bromide 5. Lead(IV) carbonate 7. Iron(III) oxide 9. lead(II) carbonate 6. Copper(II) nitrate 8. Tin(IV) fluoride 10. copper(I) sulfate . ﺍﻻﺗﻴﻨﻴﻪ ﺑﺎﻷﺮﻗﺎﻡ ﺍﻟﺘﺄﻜﺴﺪ ﺭﻗﻢ ﻳﻜﺘﺐ ﺍﻟﺘﺄﻜﺴﺪ ﺣﺎﻻﺕ ﻣﺘﻌﺪﺩﺓ ﺍﻟﻔﻠﺰﺍﺕ : ﻣﺜﺎﻝ Iron(II) oxide Fe. O Iron(III) oxide Fe 2 O 3 31

Q 2) Write full balanced equations, including state symbols, for the following reactions: a) Hydrogen reacting with nitrogen to produce ammonia gas. b) Silver nitrate solution reacting with hydrochloric acid to give sliver chloride and nitric acid c) The decomposition of hydrogen peroxide give water and oxygen d) The reaction of carbon monoxide with iron(III) oxide to give iron and carbon dioxide. This reaction occurs at high temperatures in the blast furnace. Homework Write word and formula equations for the chemical reaction that occur when aqueous solution of sodium hydroxide is added to hydrochloric acid to give sodium chloride and water. 32

Kinetic particle theory 17. 13 Use the kinetic particle theory to explain the main characteristics of the three states of matter and changes between the states: • the basic assumptions of the kinetic theory as applied to an ideal gas; • the liquid state, including melting, vaporisation and vapour pressure; • the lattice structure of a crystalline solid. : ﺍﻷﺨﺺ ﻭﻋﻠﻰ ، ﺑﻴﻨﻬﺎ ﻓﻴﻤﺎ ﻭﺍﻟﺘﻐﺭﺍﺕ ﺍﻟﺜﻼﺙ ﺍﻟﻤﺎﺩﺓ ﻟﺤﺎﻻﺕ ﺍﻟﺮﺋﻴﺴﻴﺔ ﻟﺘﻔﺴﻴﺮﺍﻟﺨﺼﺎﺋﺺ ﺍﻟﺠﺰیﺌﻴﺔ ﺍﻟﺤﺮﻛﺔ ﻧﻈﺮیﺔ یﺴﺘﺨﺪﻡ 17. 13 ﻏﺎﺯﻣﺜﺎﻟﻲ؛ ﻋﻠﻰ ﻓﻴﺘﻄﺒﻴﻘﻬﺎ ﺍﻟﺠﺰیﺌﻴﺔ ﺍﻟﺤﺮﻛﺔ ﻟﻨﻈﺮیﺔ ﺍﻷﺴﺎﺳﻴﺔ • ﺍﻻﻓﺘﺮﺍﺿﺎﺕ : ﻭﺍﻟﺘﺒﺨﺮﻭﺍﻟﻀﻐﻄﺎﻟﺒﺨﺎﺭﻱ ﺍﻻﻧﺼﻬﺎﺭ ﻓﻴﻬﺎ ﺑﻤﺎ • ﺍﻟﺤﺎﻟﺓﺎﻟﺴﺎﺋﻠﺔ . ﺻﻠﺒﺔ ﺑﻠﻮﺭیﺔ ﻟﻤﺎﺩﺓ ﺍﻟﺸﺒﻜﻲ • ﺍﻟﺘﺮﻛﻴﺐ Objectives : Key vocabulary: 1. Know the basic assumptions of the kinetic theory as applied to an ideal gas. 2. Use the kinetic particle theory to explain the main characteristics of the three states of matter and changes between the states. - kinetic particle theory -ideal gas -lattice structure -crystalline solid -vaporisation a) Basic assumptions of the kinetic theory: 1. A gas has no fixed shape or volume, but always spreads out to fill any container. 2. There almost no forces of attraction between the particles so they are completely free of each other. 3. The particles are widely spaced and scattered at random throughout the container so there is no order in the system. 4. The particles move rapidly in all directions, frequently colliding with each other and the side of the container. 5. With increase in temperature, the particles move faster as they gain kinetic energy. 33

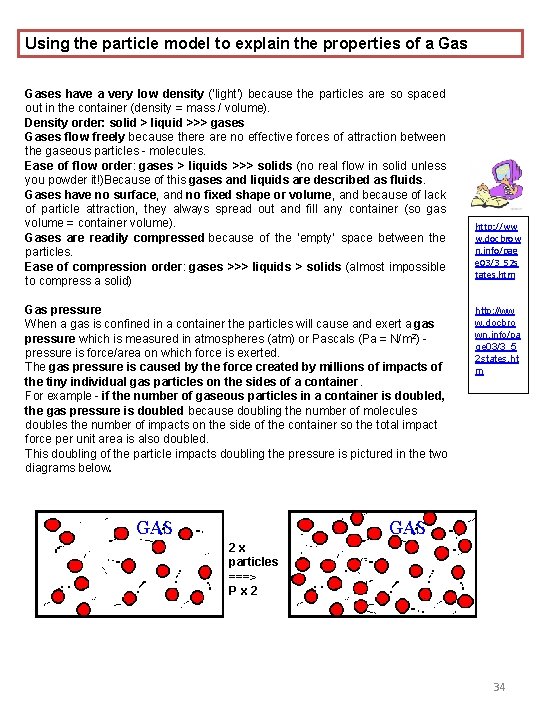
Using the particle model to explain the properties of a Gases have a very low density (‘light’) because the particles are so spaced out in the container (density = mass / volume). Density order: solid > liquid >>> gases Gases flow freely because there are no effective forces of attraction between the gaseous particles - molecules. Ease of flow order: gases > liquids >>> solids (no real flow in solid unless you powder it!)Because of this gases and liquids are described as fluids. Gases have no surface, and no fixed shape or volume, and because of lack of particle attraction, they always spread out and fill any container (so gas volume = container volume). Gases are readily compressed because of the ‘empty’ space between the particles. Ease of compression order: gases >>> liquids > solids (almost impossible to compress a solid) Gas pressure When a gas is confined in a container the particles will cause and exert a gas pressure which is measured in atmospheres (atm) or Pascals (Pa = N/m 2) - pressure is force/area on which force is exerted. The gas pressure is caused by the force created by millions of impacts of the tiny individual gas particles on the sides of a container. For example - if the number of gaseous particles in a container is doubled, the gas pressure is doubled because doubling the number of molecules doubles the number of impacts on the side of the container so the total impact force per unit area is also doubled. This doubling of the particle impacts doubling the pressure is pictured in the two diagrams below. http: //ww w. docbrow n. info/pag e 03/3_52 s tates. htm http: //ww w. docbro wn. info/pa ge 03/3_5 2 states. ht m 2 x particles ===> P x 2 34

Exercises Q 1) Draw simple diagrams to show the three states of matter? Solid particles Liquid particles Gas particles Q 2) Use assumptions of the kinetic theory and the shapes of the three states of matter to complete the following table: Property Order of particles ﺍﻟﺠﺴﻴﻤﺎﺕ ﺗﺮﺗﻴﺐ Kinetic energy ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﻄﺎﻗﺔ Intermolecular forces ﺍﻟﺠﺰﻳﺌﺎﺕ ﺑﻴﻦ ﺍﻟﺘﺠﺎﺫﺏ ﻗﻮﺓ Shape ﺍﻟﺸﻜﻞ Spacing of particles ﺍﻟﺠﺴﻴﻤﺎﺕ ﺑﻴﻦ ﺍﻟﻤﺴﺎﻓﺔ Compressibility ﺍﻟﻀﻐﻆ ﻋﻠﻰ ﺍﻟﻘﺪﺭﺓ Conduction of heat ﺍﻟﺤﺮﺍﺭﺓ ﺗﻮﺻﻴﻞ Solids Liquids Gases Q 3) Which of the three states of matter: a. Have the most ordered molecules-------b. Take the shape of the container. -------c. Have very weak forces between its molecules-------. d. Have fixed shape. -------e. Have the higher kinetic energy molecules. -------f. Have higher compressibility. ------------35