Chemistry UNIT 2 Name Date Chemistry Unit 2















- Slides: 20

Chemistry UNIT 2

Name: Date: Chemistry Unit 2 Properties of Matter

Definitions l l Physical properties can be observed without chemically changing matter. Chemical properties describe how a substance interacts with other substances.

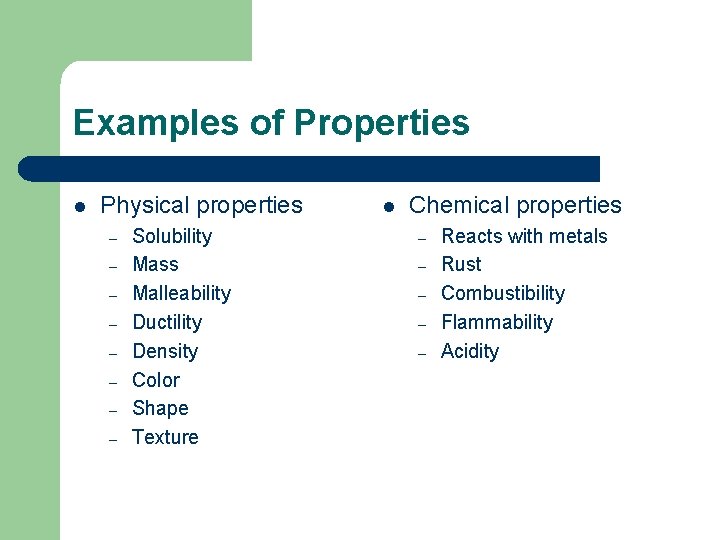
Examples of Properties l Physical properties – – – – Solubility Mass Malleability Ductility Density Color Shape Texture l Chemical properties – – – Reacts with metals Rust Combustibility Flammability Acidity

Phases of Matter l Solids have a definite shape and definite volume. – Particles are packed close together

Phases of Matter l Liquids have no shape and have a definite volume. – Particles have room to move.

Phases of Matter l Gases have no shape and no volume. – Particles are far apart from each other.

Types of Changes l l Physical Change- no change in the identity of a substance. Chemical Change- one or more new substances are produced

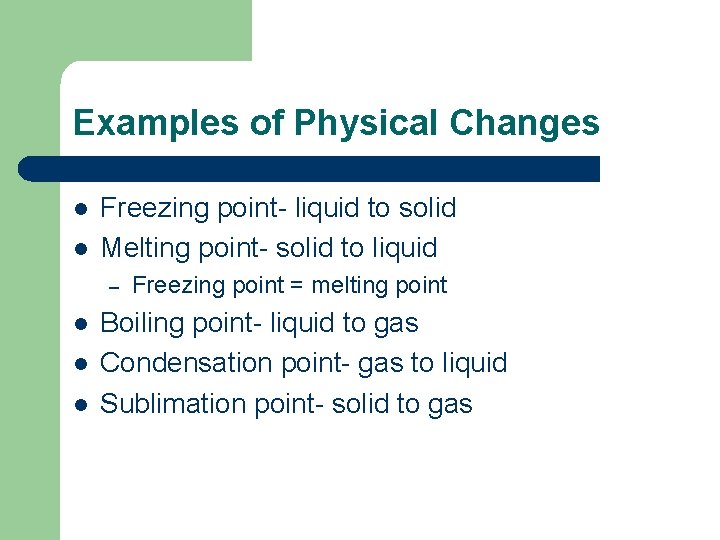
Examples of Physical Changes l l Freezing point- liquid to solid Melting point- solid to liquid – l l l Freezing point = melting point Boiling point- liquid to gas Condensation point- gas to liquid Sublimation point- solid to gas

Law of Conservation of Mass l Mass cannot be created nor destroyed.

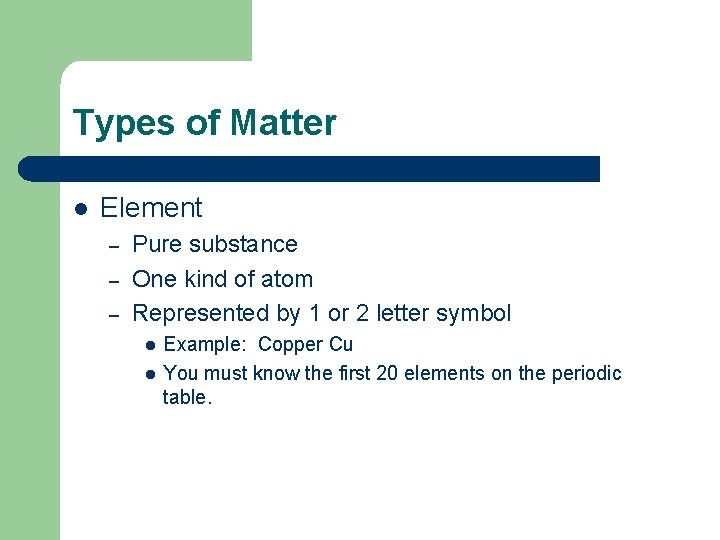
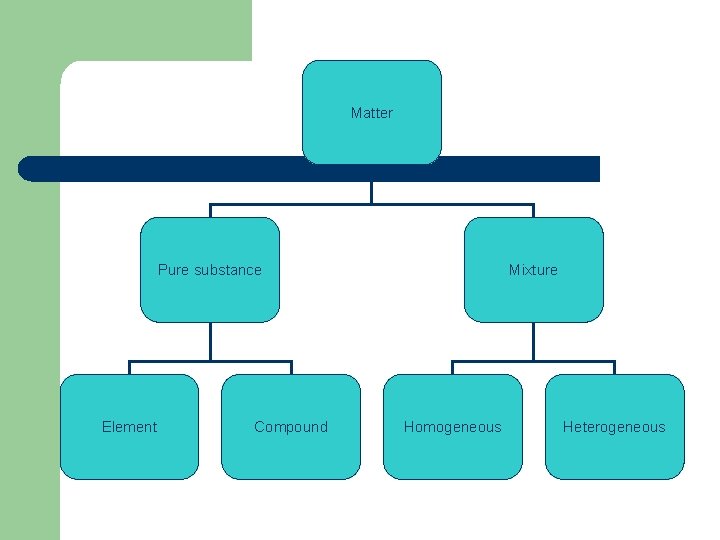
Types of Matter l Element – – – Pure substance One kind of atom Represented by 1 or 2 letter symbol l l Example: Copper Cu You must know the first 20 elements on the periodic table.

Compound l l l One or more elements chemically combined Represented by a chemical formula Ex. Water H 2 O Water can decompose into hydrogen and oxygen gas Always combine in the same proportions

Mixture l l One or more substance physically combine Do not combine in same proportions Heterogeneous- not the same and not uniform Homogeneous (solution)- same and uniform – – Alloy- mixture of two or more metals Pure substances are homogeneous

Matter Pure substance Element Compound Mixture Homogeneous Heterogeneous

Practice l l l Hydrogen peroxide Carbon dioxide Pizza Salad dressing Rust Apple juice l l l Carbon monoxide Steel Calcium lead

Separating Mixtures l The components of a mixture may be separated based on the physical properties of the mixture.

Properties l l l Magnetism Density-(an instrument used to separate mixtures when tiny particles are dissolved is a centrifuge Filtration- used to separate liquids and solids Evaporation- used to separate solutions Distillation- used to separate solutions

Density l Density- mass per unit of volume l D=m/v l Unit is g/m. L or g/cm 3

Practice l Suppose we have an object with a mass of 5 grams and a volume of 2 m. L. What is the density?

Density of Water l l The density of water is 1 g/m. L. What happens to an object whose density is greater than one?