Take on Typhoid core slides NAME TITLE DATE








- Slides: 9

Take on Typhoid core slides NAME TITLE DATE (Optional) PATH/Rocky Prajapati

Typhoid burden • Typhoid continues to be a substantial public health threat that disproportionately impacts children and marginalized populations in much of Asia and sub-Saharan Africa. • The burden of typhoid is likely underestimated due to challenges in surveillance and available diagnostics. • Current estimate is nearly 12 million cases and more than 128, 000 deaths each year. • Vaccination, along with improvements in water, sanitation, and hygiene (WASH), are key components to an integrated strategy to prevent typhoid. PAGE 2 PATH/Doune Porter.

Why TCVs? • Until recently, the two typhoid vaccines that were available were underutilized in high-burden countries despite typhoid’s substantial and detrimental impact and World Health Organization (WHO) recommendation for their use. • New typhoid conjugate vaccines (TCVs) have the potential to overcome many challenges that have impeded the uptake of earlier vaccines through: • • • PAGE 3 Longer-lasting protection; Suitability for children under the age of two, and; Easier inclusion in routine immunization programs. PATH/Rocky Prajapati.

Building momentum around recent developments In the past few months, several milestones for global TCV policies have been reached: • October 2017: WHO’s Strategic Advisory Group of Experts (SAGE) on Immunization recommended introduction of TCVs in typhoid-endemic countries. • November 2017: Gavi, the Vaccine Alliance announced US$85 million in funding to support TCV introduction in Gavi-eligible countries. • December 2017: WHO prequalified the first TCV, Typbar. TCV®. • March 2018: New WHO position paper recommending routine introduction of TCVs in typhoid-endemic countries. • April 2018: Gavi application available for countries to apply for support to introduce TCV. PAGE 4 Bill & Melinda Gates Foundation/Sam Reinders.

Typbar-TCV® • Typbar-TCV, manufactured by Bharat Biotech, is the first typhoid conjugate vaccine to be prequalified by WHO. • It is approved as a single dose for adults, children, and infants 6 months of age and older. • First typhoid vaccine to be approved for children younger than 2 years. • Offers protection for at least 3 years. Additional studies are needed to determine the duration of protection and need for booster doses. • Studies have shown that Typbar-TCV elicits a strong immune response in infants and adults. PAGE 5 Bill & Melinda Gates Foundation/Sam Reinders.

Now is the time to take on typhoid We currently face a triple threat that elevates the urgency for better prevention and control of typhoid: 1. Urbanization: Rapid urbanization is leading to overcrowded populations in cities across Asia and sub-Saharan Africa that often have outdated, inadequate, or unsafe sanitation systems, increasing the risk of typhoid transmission. 2. Climate change: Higher likelihood for natural disasters to occur. • During droughts, shallow water sources are more likely to be contaminated with typhoid; flooding can overwhelm sewage systems or sanitation facilities, allowing human waste to contaminate water sources. 3. Drug resistance: Drug- and multidrug-resistant strains of typhoid are spreading and becoming more difficult to treat. PAGE 6 PATH/Monique Berlier.

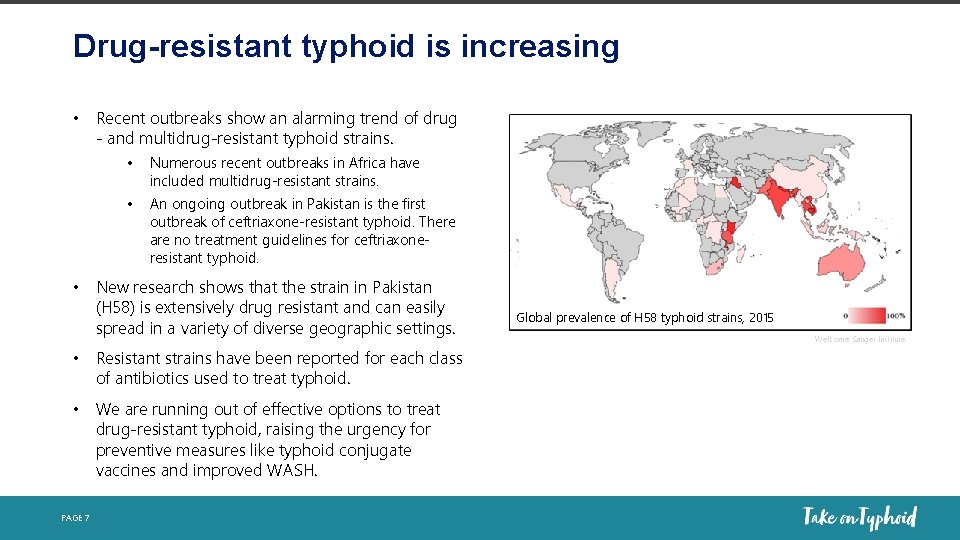
Drug-resistant typhoid is increasing • Recent outbreaks show an alarming trend of drug - and multidrug-resistant typhoid strains. • Numerous recent outbreaks in Africa have included multidrug-resistant strains. • An ongoing outbreak in Pakistan is the first outbreak of ceftriaxone-resistant typhoid. There are no treatment guidelines for ceftriaxoneresistant typhoid. • New research shows that the strain in Pakistan (H 58) is extensively drug resistant and can easily spread in a variety of diverse geographic settings. • Resistant strains have been reported for each class of antibiotics used to treat typhoid. • We are running out of effective options to treat drug-resistant typhoid, raising the urgency for preventive measures like typhoid conjugate vaccines and improved WASH. PAGE 7 Global prevalence of H 58 typhoid strains, 2015 Wellcome Sanger Institute.

Learn more at: http: //takeontyphoid. org PATH/Rocky Prajapati

Take on Typhoid partnership • In September 2017, Ty. VAC and the Coalition against Typhoid joined forces to launch the Take On Typhoid partnership. • Building on the momentum, experience, and expertise of diverse partners, Take On Typhoid focuses attention on typhoid and the need for typhoid conjugate vaccines and water, sanitation, and hygiene to reduce the burden and impact of typhoid fever. • Visit the www. takeontyphoid. org website for advocacy resources, blogs, publications, and more. PAGE 9 PATH/Georgina Goodwin.