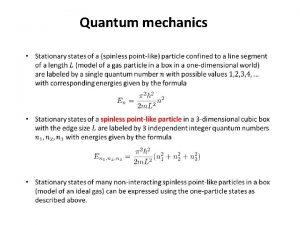
Advanced Chemistry Unit 6 Quantum Mechanics Advanced Chemistry














![Figure out the shortcut for 51 Sb using the table [Kr] 5 s 2 Figure out the shortcut for 51 Sb using the table [Kr] 5 s 2](https://slidetodoc.com/presentation_image_h2/25f10ccb50897f75da13e13f25d72e7a/image-76.jpg)



- Slides: 80

Advanced Chemistry Unit 6 Quantum Mechanics

Advanced Chemistry Unit 6 Quantum Mechanics Things aren’t always as they seem…

Remember, Protons determine which element it is…. But Electrons determine what an element will do! In this unit, we will take a closer look at how electrons behave…. . Quantum Mechanics

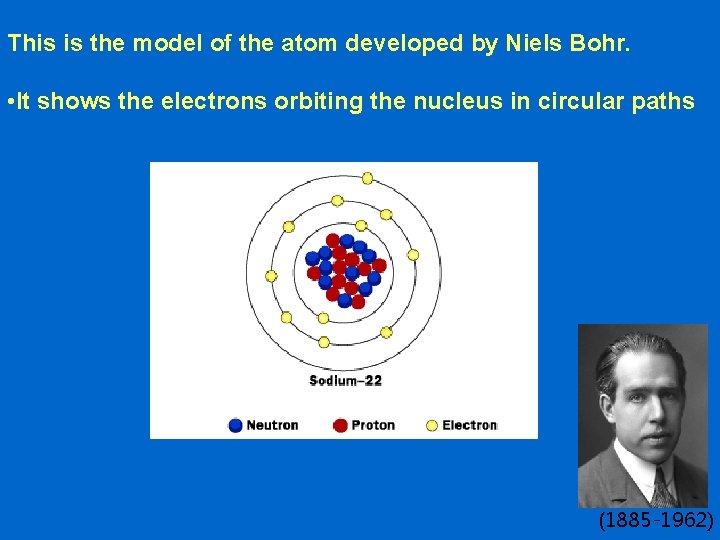
This is the model of the atom developed by Niels Bohr. • It shows the electrons orbiting the nucleus in circular paths (1885 -1962)

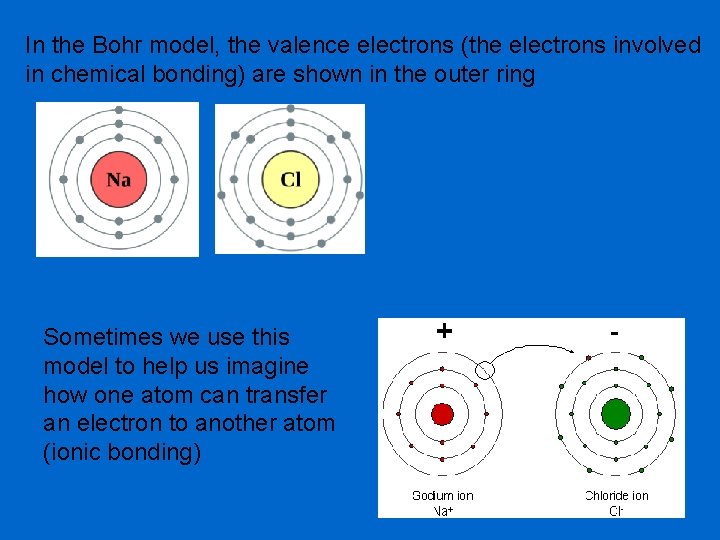
In the Bohr model, the valence electrons (the electrons involved in chemical bonding) are shown in the outer ring Sometimes we use this model to help us imagine how one atom can transfer an electron to another atom (ionic bonding)

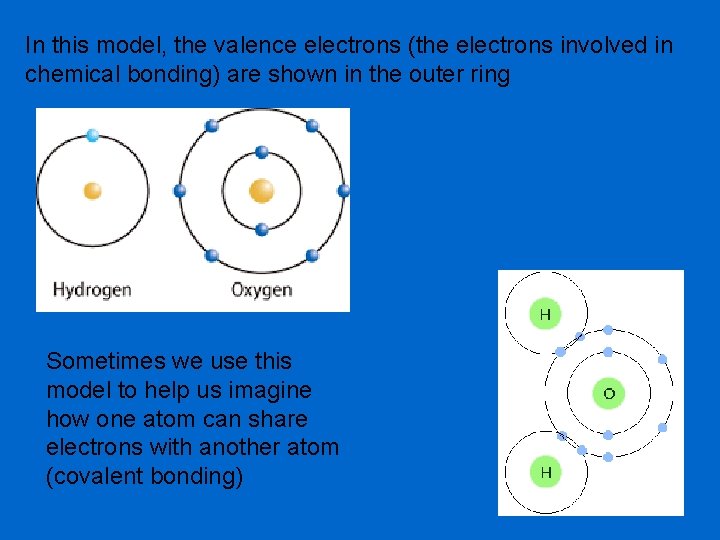
In this model, the valence electrons (the electrons involved in chemical bonding) are shown in the outer ring Sometimes we use this model to help us imagine how one atom can share electrons with another atom (covalent bonding)

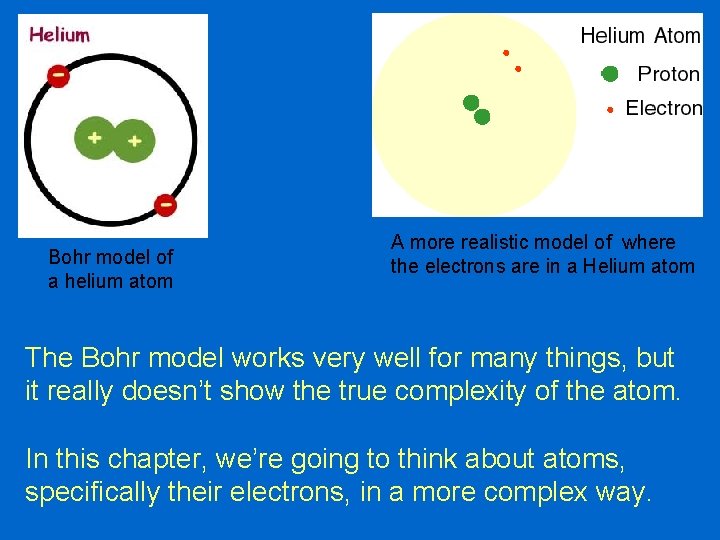
Bohr model of a helium atom A more realistic model of where the electrons are in a Helium atom The Bohr model works very well for many things, but it really doesn’t show the true complexity of the atom. In this chapter, we’re going to think about atoms, specifically their electrons, in a more complex way.

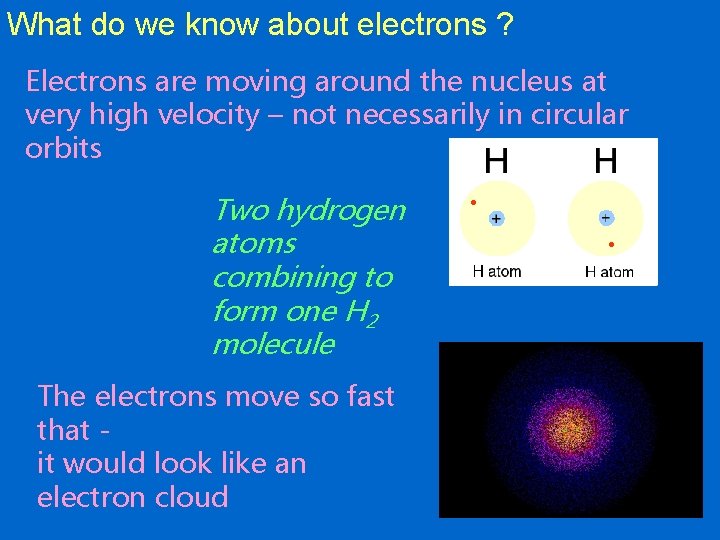
What do we know about electrons ? Electrons are moving around the nucleus at very high velocity – not necessarily in circular orbits Two hydrogen atoms combining to form one H 2 molecule The electrons move so fast that it would look like an electron cloud

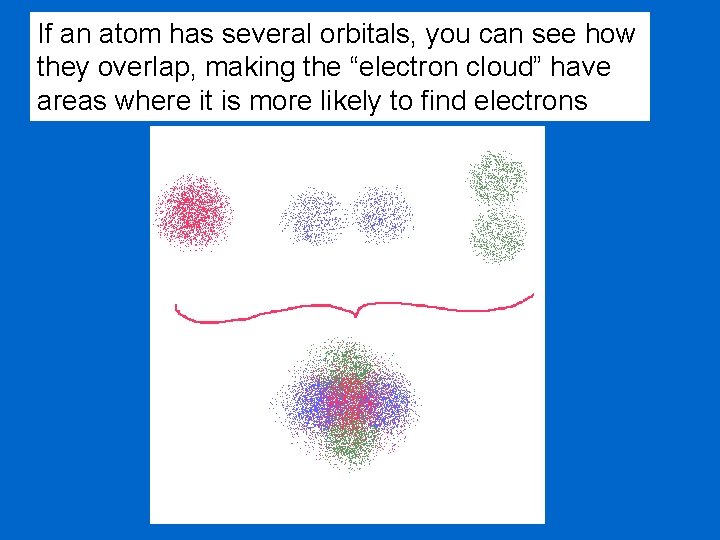
Within the electron cloud, there areas where you are more likely to find an electron and areas where finding an electron is less likely. Mathematicians call this “probability” An area where you are likely to find electrons is called an orbital. We will look at the different types of orbitals in a moment. But first, an analogy.

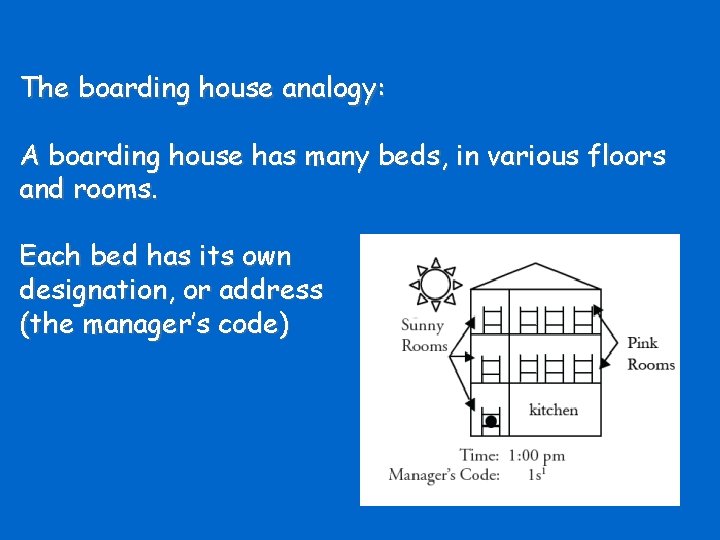
The boarding house analogy: A boarding house has many beds, in various floors and rooms. Each bed has its own designation, or address (the manager’s code)

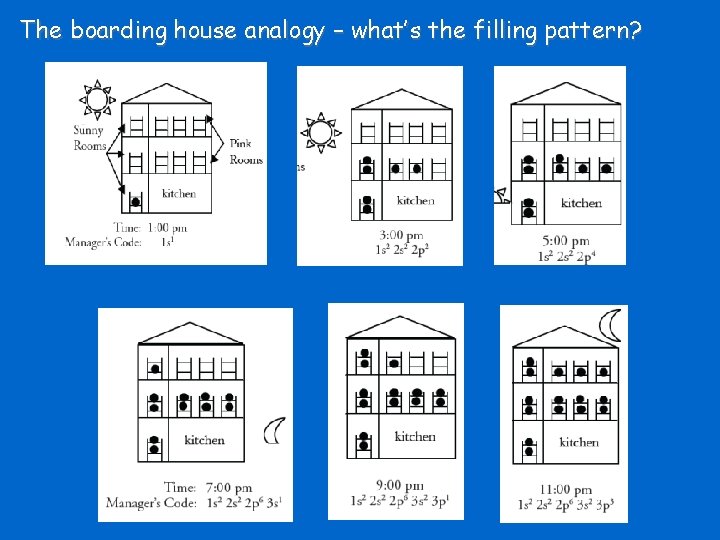
The boarding house analogy – what’s the filling pattern?

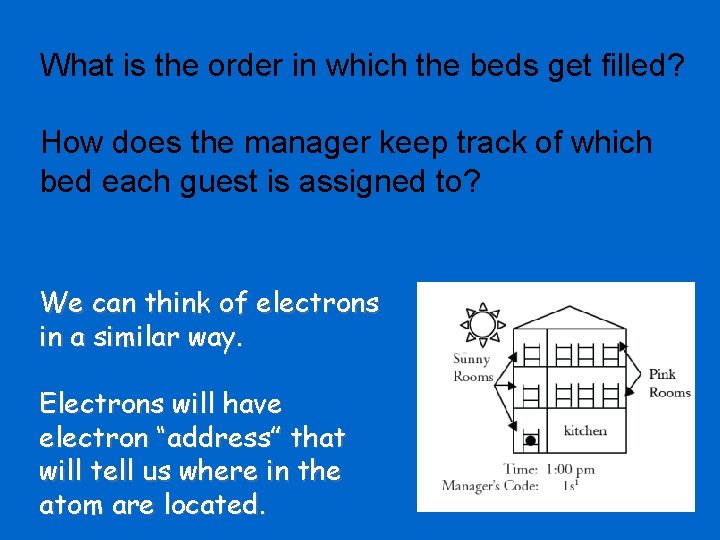
What is the order in which the beds get filled? How does the manager keep track of which bed each guest is assigned to? We can think of electrons in a similar way. Electrons will have electron “address” that will tell us where in the atom are located.

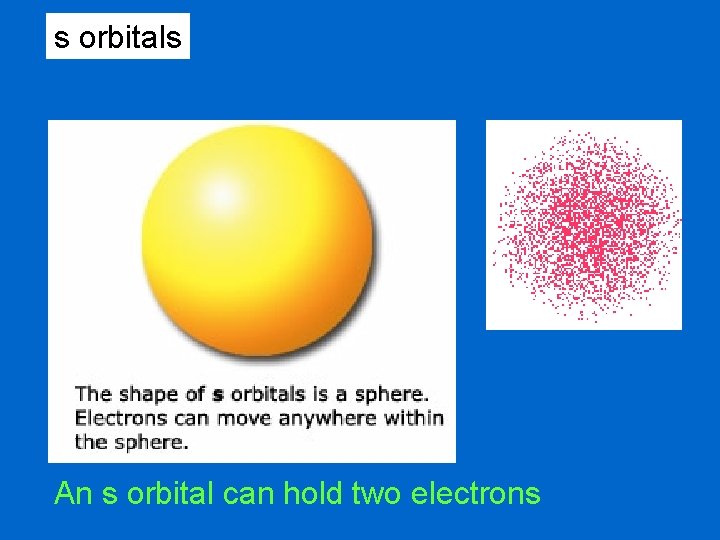
s orbitals An s orbital can hold two electrons

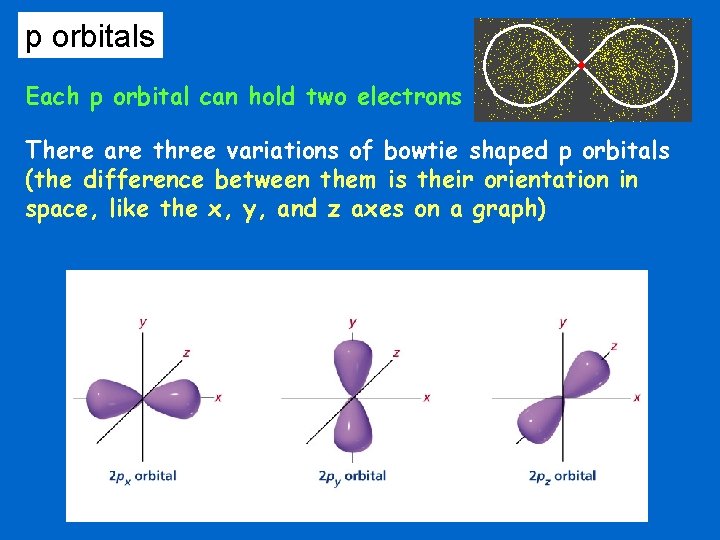
p orbitals Each p orbital can hold two electrons There are three variations of bowtie shaped p orbitals (the difference between them is their orientation in space, like the x, y, and z axes on a graph)

d orbitals really have interesting shapes…… “double dumbells” …and a “dumbell with a donut”! No, you don’t have to remember these shapes

f orbitals No, you don’t have to remember these shapes either

If an atom has several orbitals, you can see how they overlap, making the “electron cloud” have areas where it is more likely to find electrons

Ok, this is complicated… What do I really need to know about electron orbitals? Orbitals areas where electrons are likely to be found Orbitals have different shapes (s=sphere, p=bowtie) The more electrons an atom has, the more places (orbitals) it will need to hold the electrons Showing where the electrons are located is like a game, and it is easy if you follow the rules….

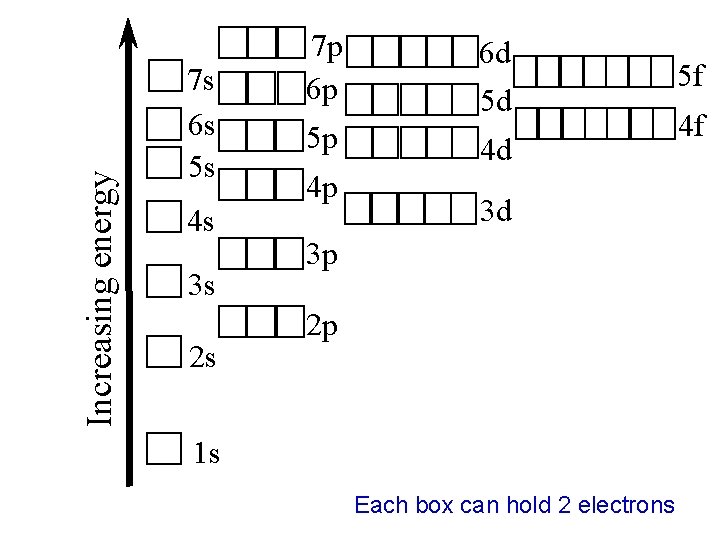
Electron Orbital Notation Ø Determine the number of electrons in the atom or ion Ø Start with the 1 s orbital and fill each orbital according to the guide (Aufbau principle) Ø Show each electron as an arrow Ø Add arrows (electrons) individually to the boxes until all electrons in the atom have been drawn Ø Follow Hund’s rule and Pauli’s principle (more on this as we do the practice atoms)

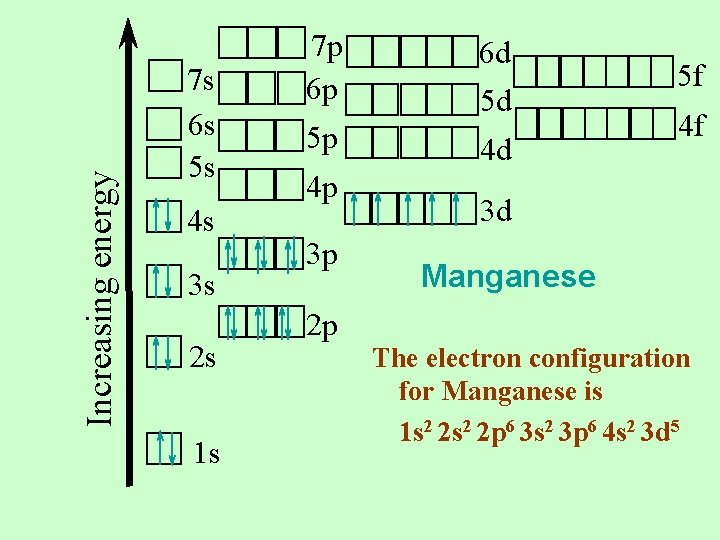
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 7 p 6 p 5 p 4 p 6 d 5 d 4 d 3 d 3 p 2 p 1 s Each box can hold 2 electrons 5 f 4 f

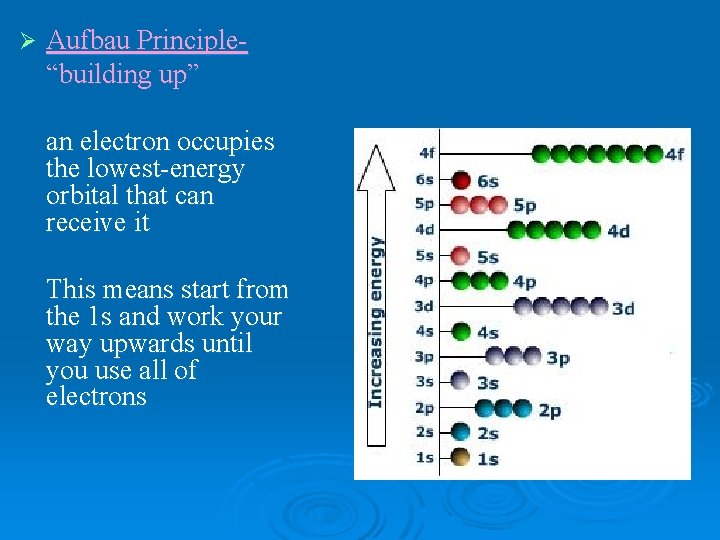
Ø Aufbau Principle“building up” an electron occupies the lowest-energy orbital that can receive it This means start from the 1 s and work your way upwards until you use all of electrons

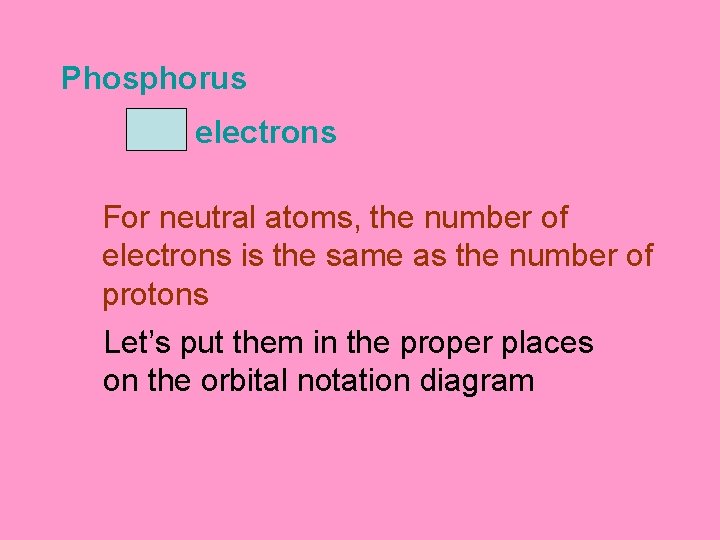
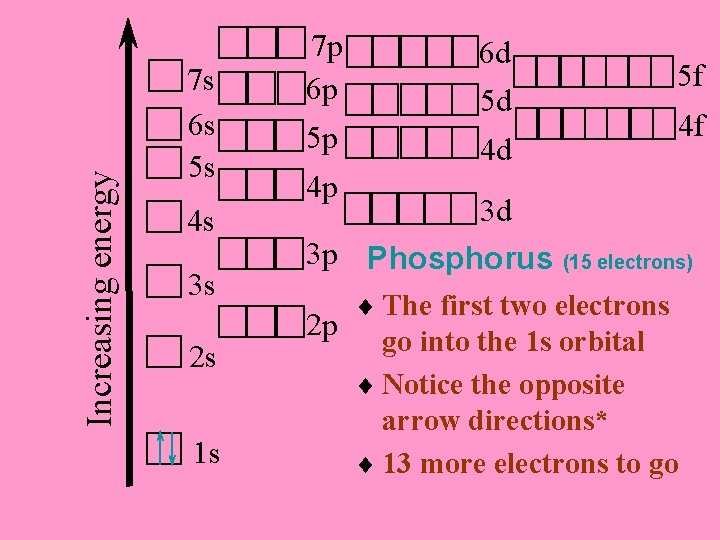
Phosphorus 15 electrons For neutral atoms, the number of electrons is the same as the number of protons Let’s put them in the proper places on the orbital notation diagram

Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 5 p 6 d 5 d 4 d 5 f 4 f 4 p 3 d 3 p Phosphorus (15 electrons) 2 p ¨ The first two electrons go into the 1 s orbital ¨ Notice the opposite arrow directions* ¨ 13 more electrons to go

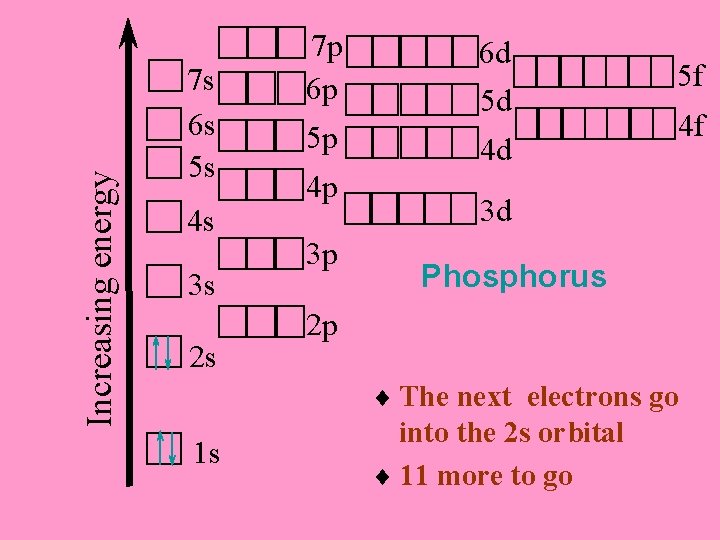
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 7 p 6 p 5 p 4 p 3 p 6 d 5 d 4 d 5 f 4 f 3 d Phosphorus 2 p ¨ The next electrons go 1 s into the 2 s orbital ¨ 11 more to go

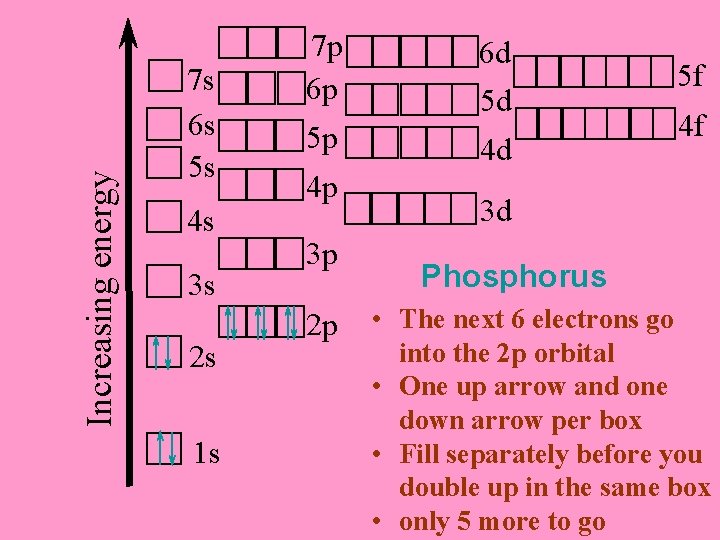
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 5 p 4 p 3 p 2 p 6 d 5 d 4 d 5 f 4 f 3 d Phosphorus • The next 6 electrons go into the 2 p orbital • One up arrow and one down arrow per box • Fill separately before you double up in the same box • only 5 more to go

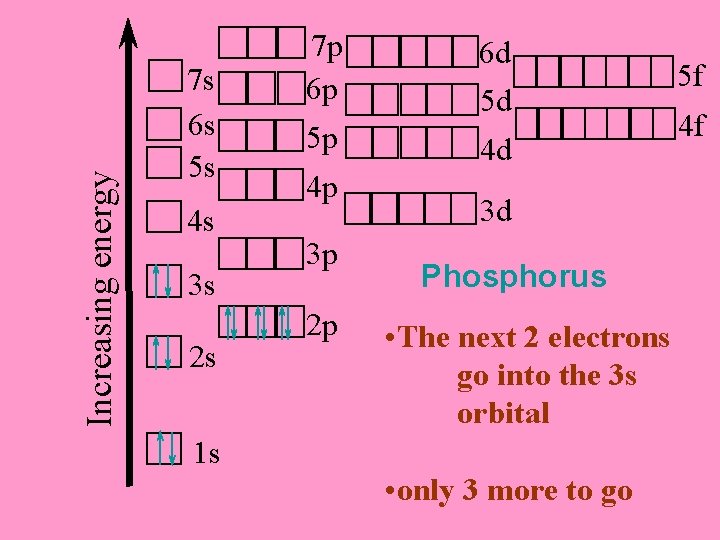
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 7 p 6 p 5 p 4 p 3 p 2 p 6 d 5 d 4 d 3 d Phosphorus • The next 2 electrons go into the 3 s orbital 1 s • only 3 more to go 5 f 4 f

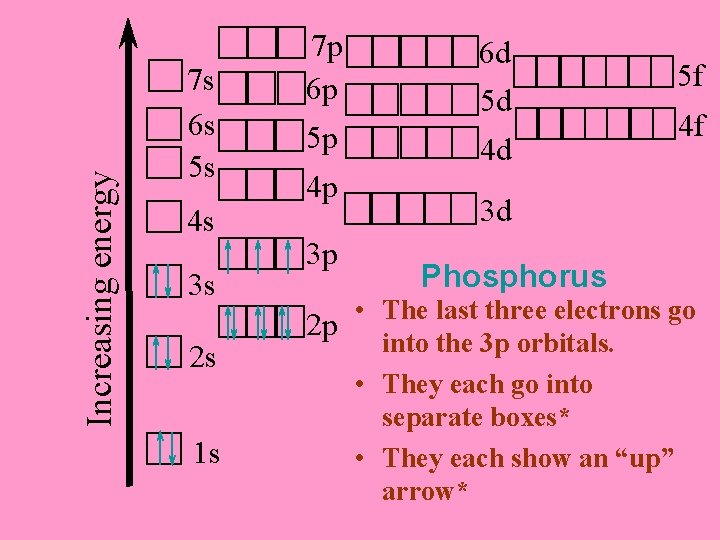
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 5 p 4 p 3 p 6 d 5 d 4 d 5 f 4 f 3 d Phosphorus • The last three electrons go 2 p into the 3 p orbitals. • They each go into separate boxes* • They each show an “up” arrow*

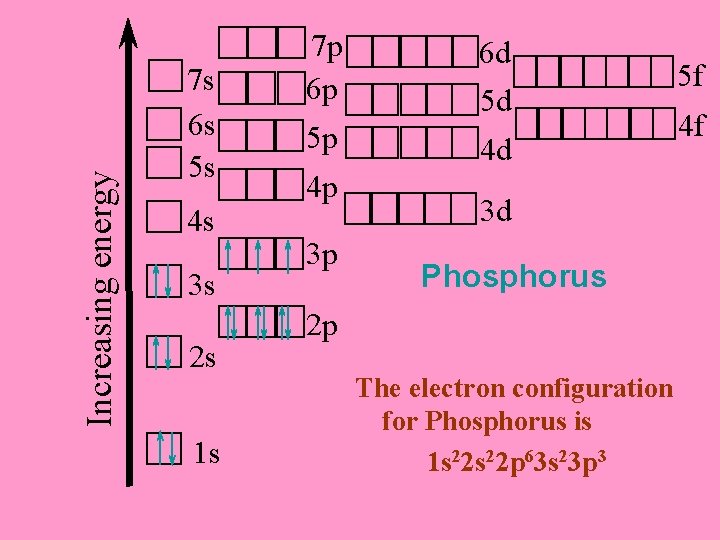
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 5 p 4 p 3 p 6 d 5 d 4 d 3 d Phosphorus 2 p The electron configuration for Phosphorus is 1 s 22 p 63 s 23 p 3 5 f 4 f

Why did we put one arrow up and one arrow down in each box? Pauli Exclusion Principle- just like no two houses can have the same address, no two electrons in the same atom can have the same “address”. If there are two electrons in the same orbital, we need to make one and the other (by tradition, we write the up arrow first)

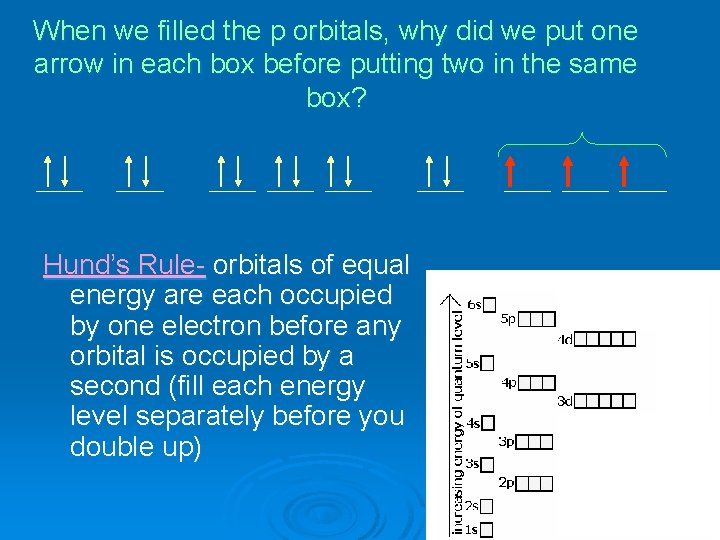
When we filled the p orbitals, why did we put one arrow in each box before putting two in the same box? Hund’s Rule- orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second (fill each energy level separately before you double up)

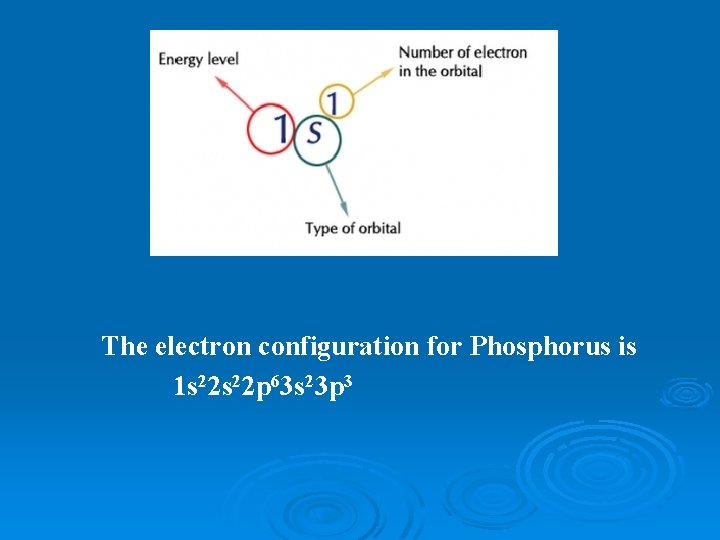
The electron configuration for Phosphorus is 1 s 22 p 63 s 23 p 3

I electron configuration! http: //www. youtube. com/watch? v=Vb 6 k. Axw. SWg. U

Orbital Diagrams and Electron Configuration ws 1 -12

Manganese 25 electrons For elements, the number of electrons is the same as the number of protons Let’s put them in the proper places on the orbital notation diagram

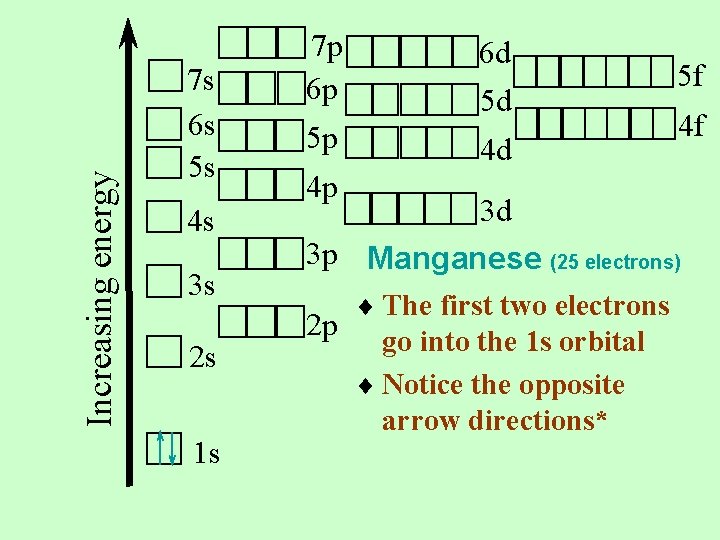
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 5 p 6 d 5 d 4 d 4 p 5 f 4 f 3 d 3 p Manganese (25 electrons) 2 p ¨ The first two electrons go into the 1 s orbital ¨ Notice the opposite arrow directions*

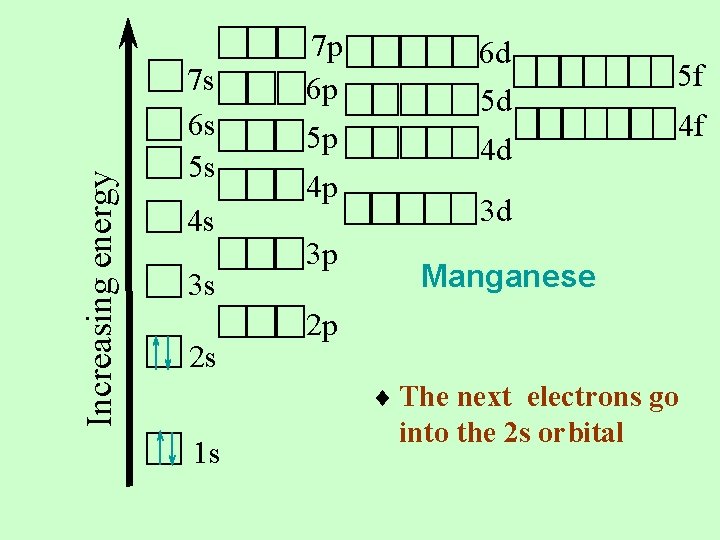
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 7 p 6 p 5 p 4 p 3 p 6 d 5 d 4 d 5 f 4 f 3 d Manganese 2 p ¨ The next electrons go 1 s into the 2 s orbital

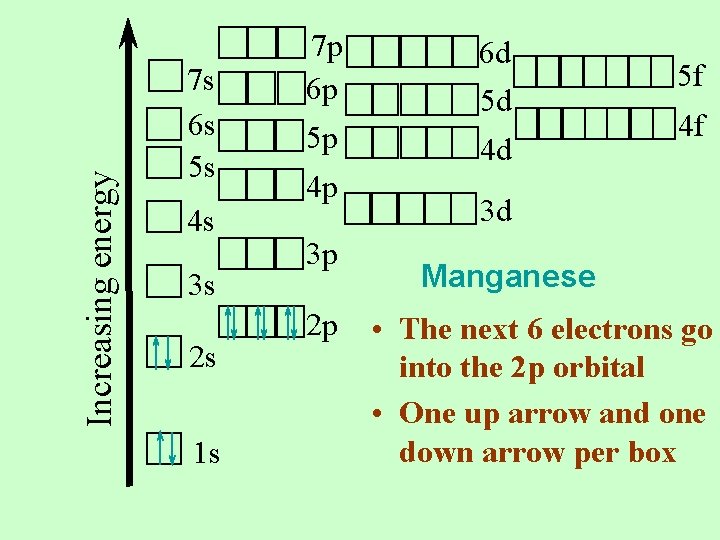
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 5 p 4 p 3 p 2 p 6 d 5 d 4 d 5 f 4 f 3 d Manganese • The next 6 electrons go into the 2 p orbital • One up arrow and one down arrow per box

Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 5 p 4 p 3 p 2 p 6 d 5 d 4 d 3 d Manganese • The next 2 electrons go into the 3 s orbital 5 f 4 f

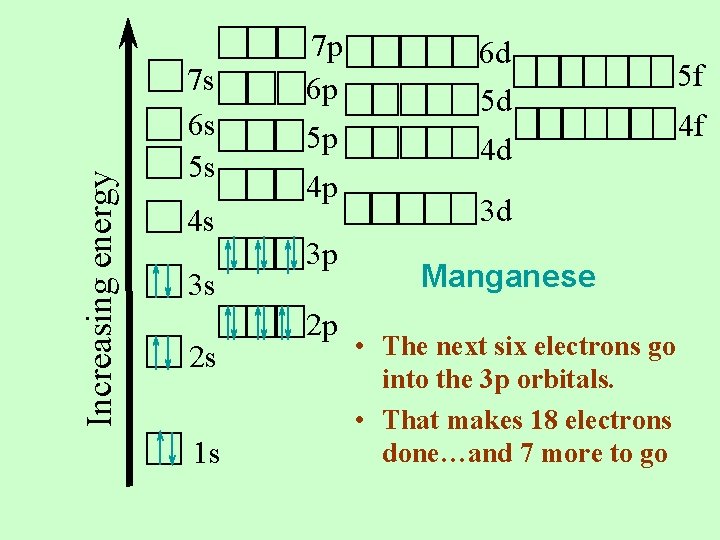
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 5 p 4 p 3 p 2 p 6 d 5 d 4 d 3 d Manganese • The next six electrons go into the 3 p orbitals. • That makes 18 electrons done…and 7 more to go 5 f 4 f

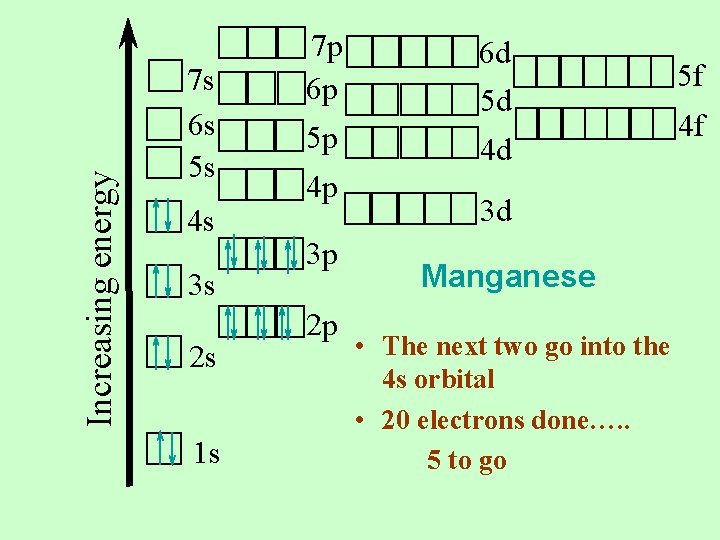
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 5 p 4 p 3 p 2 p 6 d 5 d 4 d 3 d Manganese • The next two go into the 4 s orbital • 20 electrons done…. . 5 to go 5 f 4 f

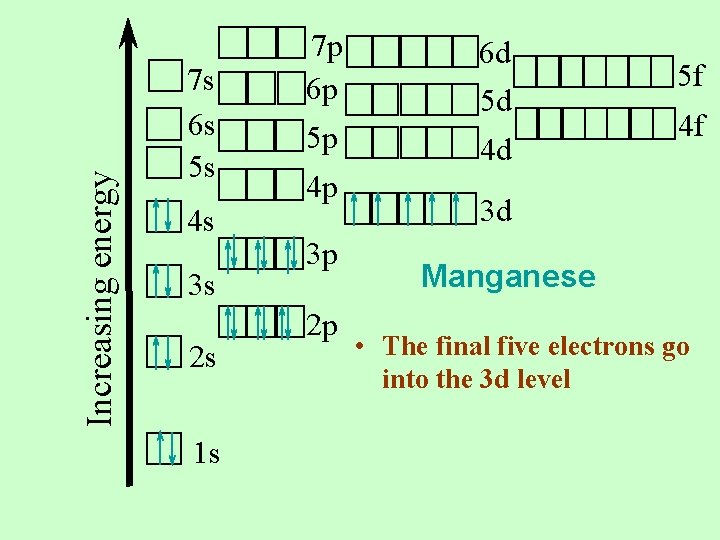
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 5 p 4 p 3 p 2 p 6 d 5 d 4 d 5 f 4 f 3 d Manganese • The final five electrons go into the 3 d level

Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 5 p 4 p 3 p 6 d 5 d 4 d 5 f 4 f 3 d Manganese 2 p The electron configuration for Manganese is 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 5

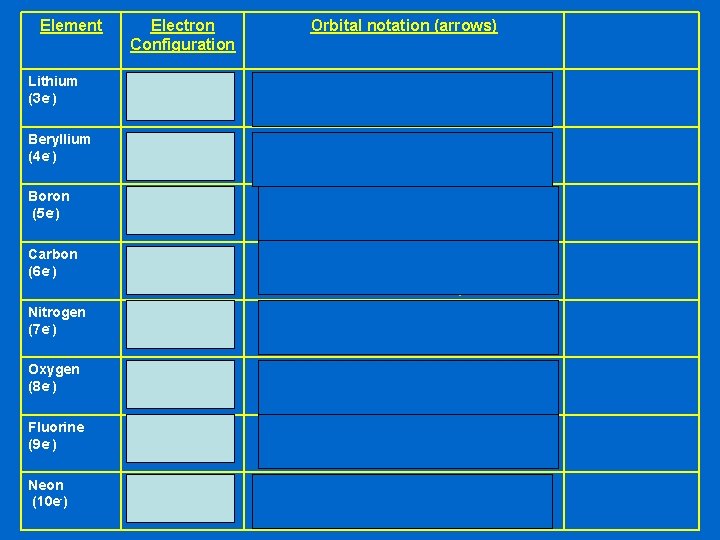
Let’s try some more Li Be B C N O F Ne This time we’ll save space and show the boxes as lines instead

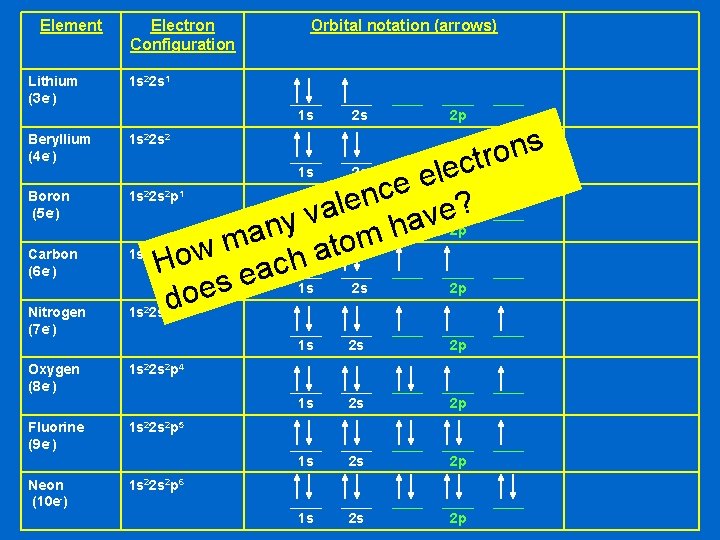
Element Electron Configuration Lithium (3 e-) 1 s 22 s 1 Beryllium (4 e-) 1 s 22 s 2 Boron (5 e-) 1 s 22 s 2 p 1 Carbon (6 e-) 1 s 22 s 2 p 2 Nitrogen (7 e-) 1 s 22 s 2 p 3 Oxygen (8 e-) 1 s 22 s 2 p 4 Fluorine (9 e-) 1 s 22 s 2 p 5 Neon (10 e-) 1 s 22 s 2 p 6 Orbital notation (arrows) ____ 1 s ____ 2 s ____ ____ 2 p ____ ____ 1 s ____ 2 s ____ ____ 2 p ____

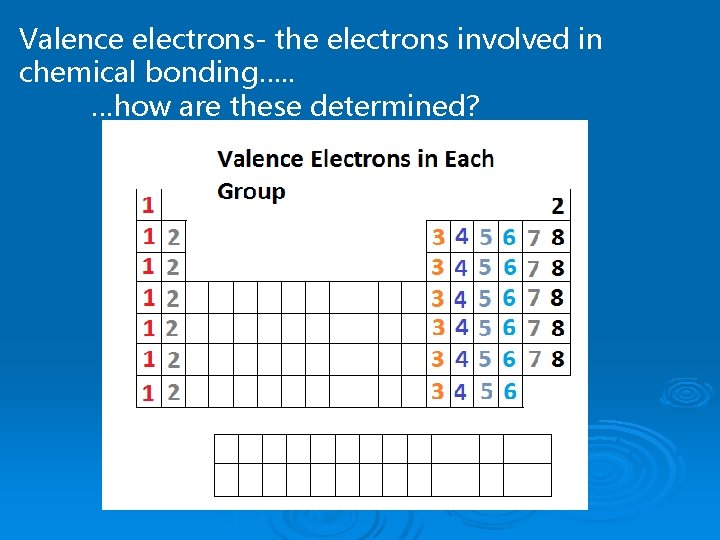
Valence electrons- the electrons involved in chemical bonding…. . …how are these determined?

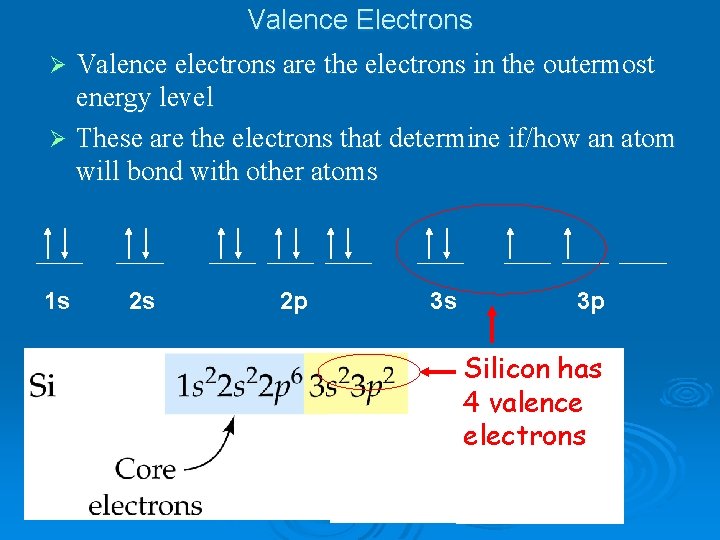
Valence Electrons Valence electrons are the electrons in the outermost energy level Ø These are the electrons that determine if/how an atom will bond with other atoms Ø 1 s 2 s 2 p 3 s 3 p Silicon has 4 valence electrons

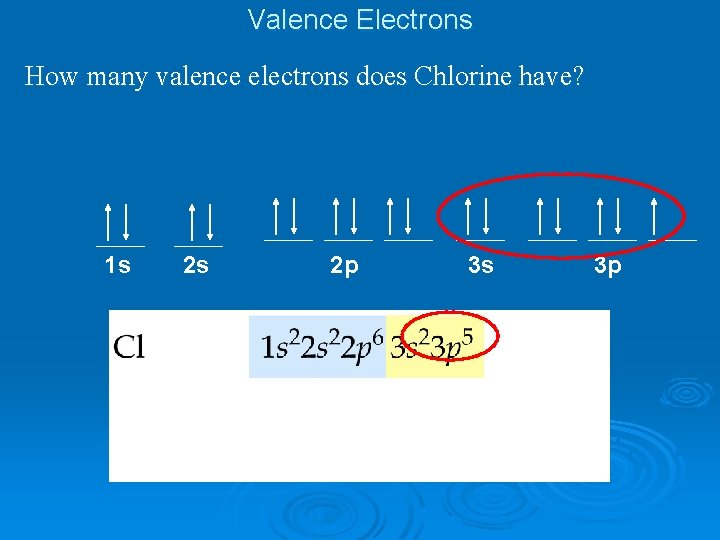
Valence Electrons How many valence electrons does Chlorine have? 1 s 2 s 2 p 3 s 3 p

Valence Electrons Can you locate the valence electrons in this atom of Cobalt ? Valence electrons are the electrons in the outermost energy level Ø These are the electrons that determine if/how an atom will bond with other atoms Ø

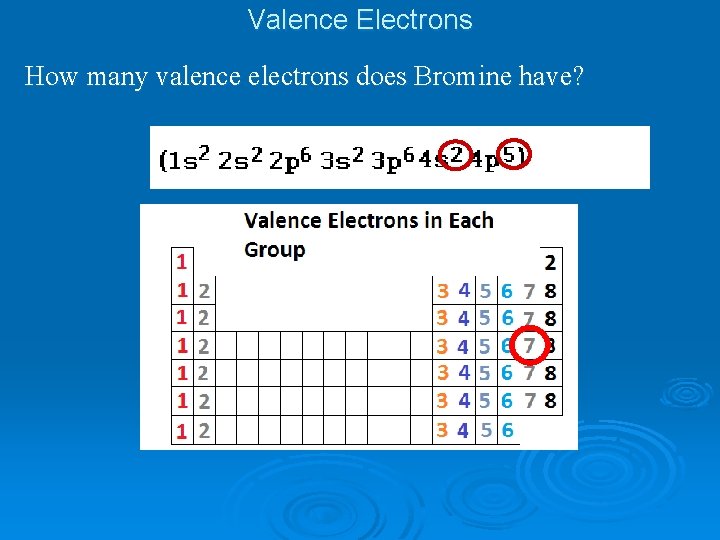
Valence Electrons How many valence electrons does Bromine have?

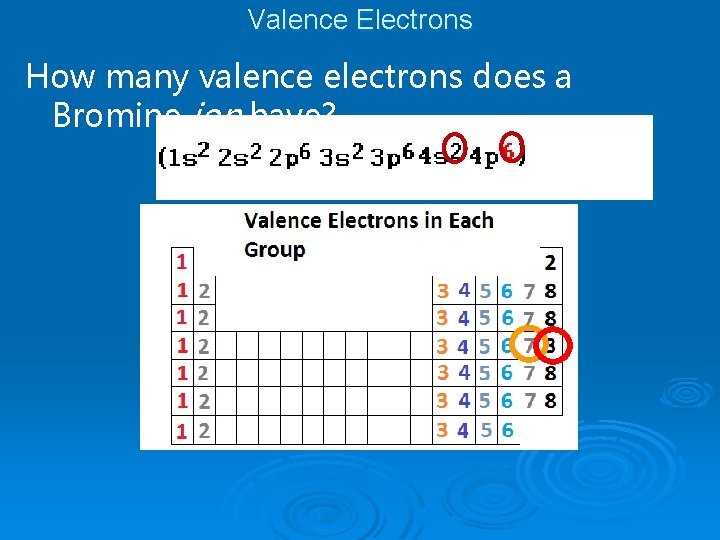
Valence Electrons How many valence electrons does a Bromine ion have?

Element Electron Configuration Lithium (3 e-) 1 s 22 s 1 Beryllium (4 e-) 1 s 22 s 2 Boron (5 e-) Carbon (6 e-) Nitrogen (7 e-) Orbital notation (arrows) ____ 1 s ____ 2 s ____ 2 p ____ s n ____ o r t c e l e e c 1 s 2 s p n ____ e e l ? ____ a v v y 1 s 2 s ha 2 p n a m m o t 1 s 2 s p w a h ____ Ho eac____ 1 s 2 s 2 p s e o 1 s 2 s d p 2 2 1 2 2 2 3 Oxygen (8 e-) 1 s 22 s 2 p 4 Fluorine (9 e-) 1 s 22 s 2 p 5 Neon (10 e-) 1 s 22 s 2 p 6 ____ 1 s ____ 2 s ____ ____ 2 p ____ 1 s ____ 2 s ____ 2 p ____

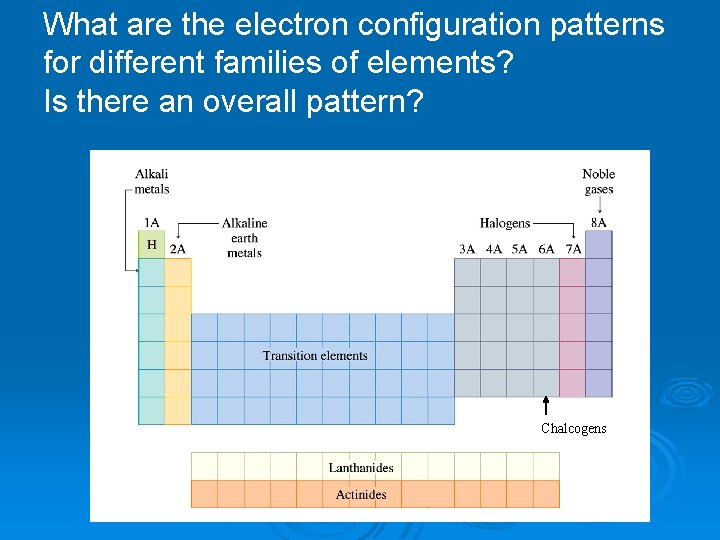
What are the electron configuration patterns for different families of elements? Is there an overall pattern? Chalcogens

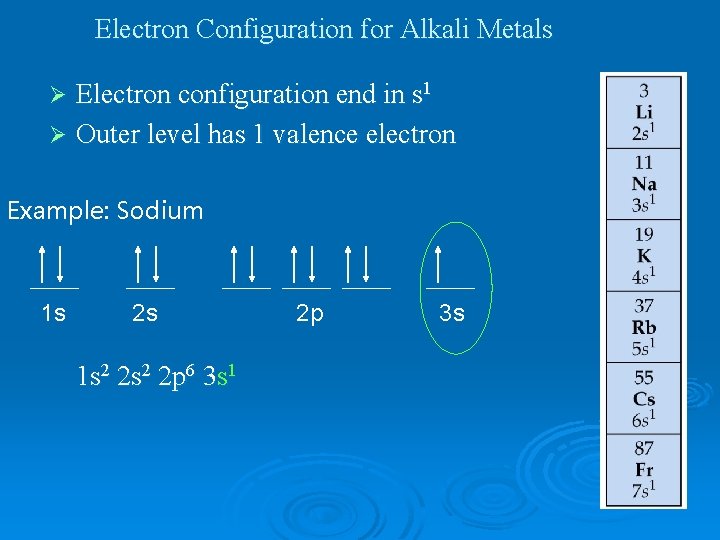
Electron Configuration for Alkali Metals Electron configuration end in s 1 Ø Outer level has 1 valence electron Ø Example: Sodium 1 s 2 s 1 s 2 2 p 6 3 s 1 2 p 3 s

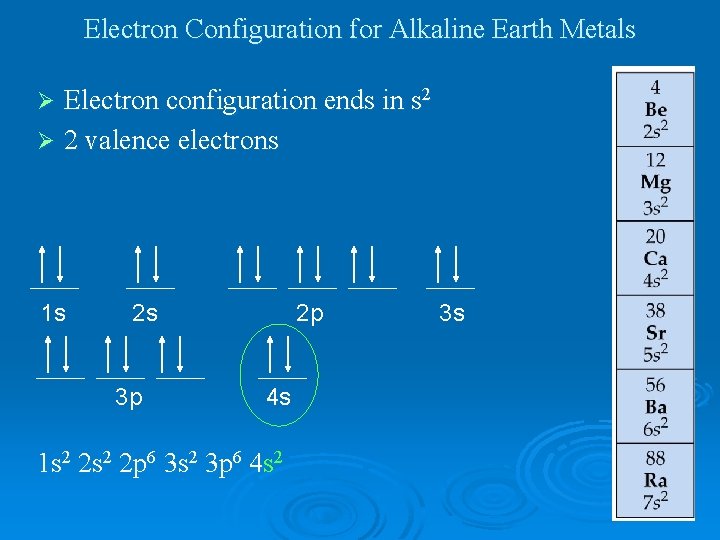
Electron Configuration for Alkaline Earth Metals Electron configuration ends in s 2 Ø 2 valence electrons Ø 1 s 2 s 3 p 2 p 4 s 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 s

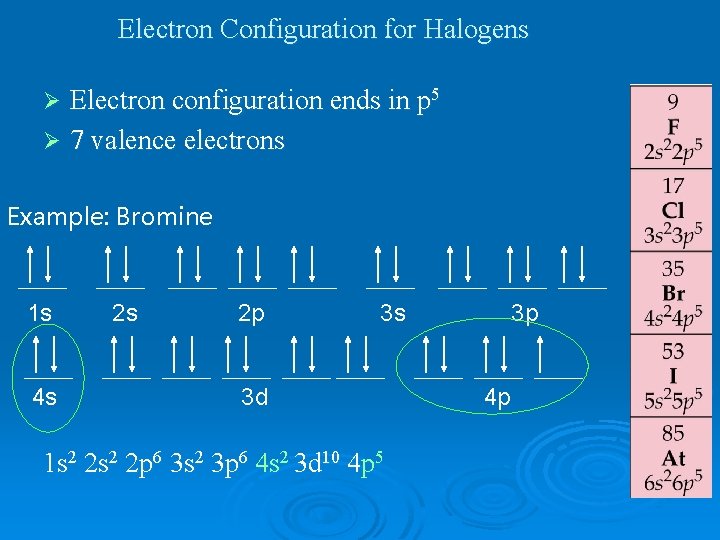
Electron Configuration for Halogens Electron configuration ends in p 5 Ø 7 valence electrons Ø Example: Bromine 1 s 4 s 2 s 2 p 3 s 3 d 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5 3 p 4 p

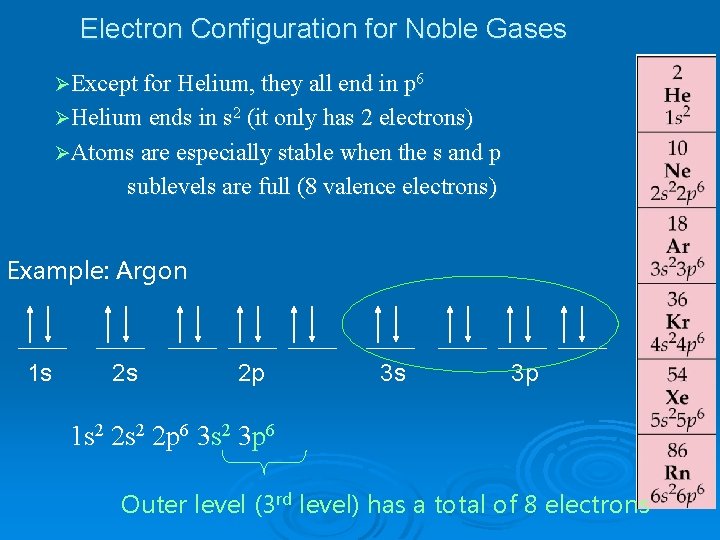
Electron Configuration for Noble Gases ØExcept for Helium, they all end in p 6 ØHelium ends in s 2 (it only has 2 electrons) ØAtoms are especially stable when the s and p sublevels are full (8 valence electrons) Example: Argon 1 s 2 s 2 p 3 s 3 p 1 s 2 2 p 6 3 s 2 3 p 6 Outer level (3 rd level) has a total of 8 electrons


Orbital Diagrams and Electron Configuration ws 13 -40

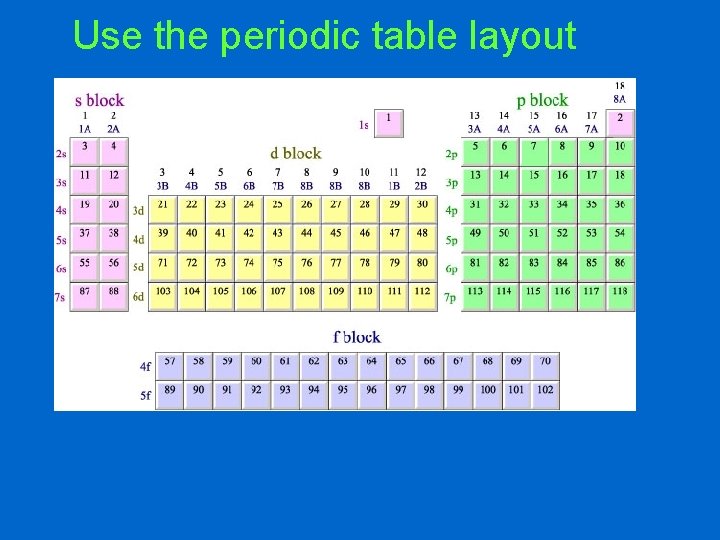
Even if you don’t have the electron filling cheat sheet, there are two other ways to figure out the electron configuration: • Create your own cheat sheet • Use the layout of the periodic table

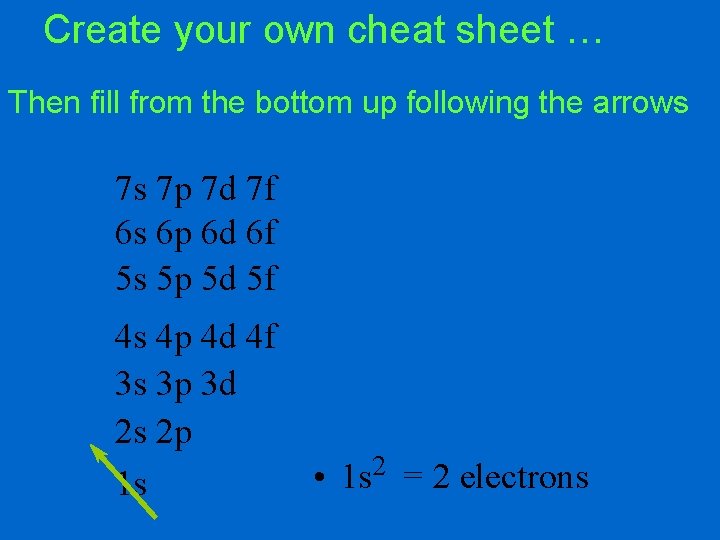
Create your own cheat sheet … (1 -7 spdf) 7 s 7 p 7 d 7 f 6 s 6 p 6 d 6 f 5 s 5 p 5 d 5 f 4 s 4 p 4 d 4 f 3 s 3 p 3 d 2 s 2 p 1 s

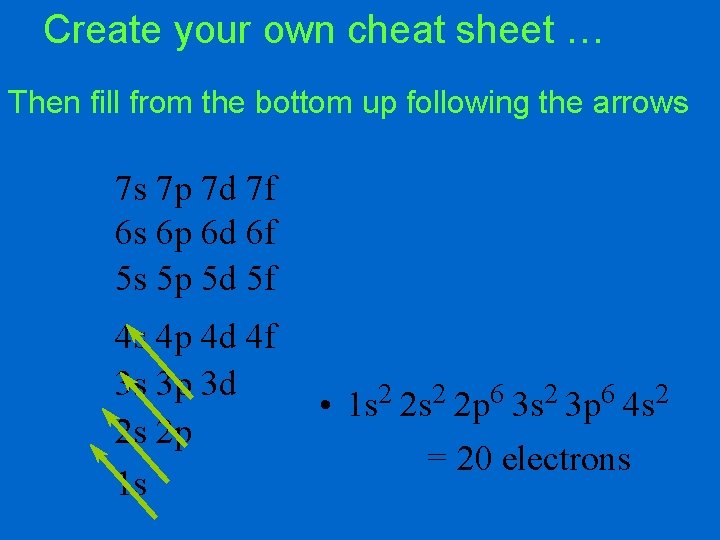
Create your own cheat sheet … Then fill from the bottom up following the arrows 7 s 7 p 7 d 7 f 6 s 6 p 6 d 6 f 5 s 5 p 5 d 5 f 4 s 4 p 4 d 4 f 3 s 3 p 3 d 2 s 2 p 1 s • 2 1 s = 2 electrons

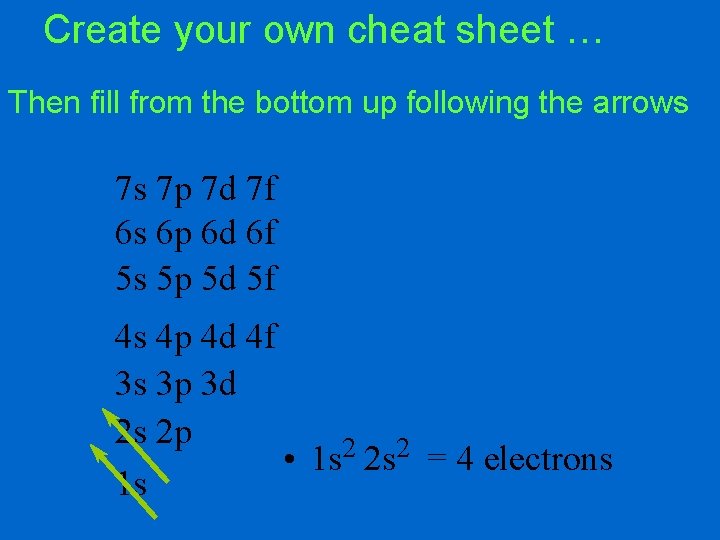
Create your own cheat sheet … Then fill from the bottom up following the arrows 7 s 7 p 7 d 7 f 6 s 6 p 6 d 6 f 5 s 5 p 5 d 5 f 4 s 4 p 4 d 4 f 3 s 3 p 3 d 2 s 2 p • 1 s 2 2 s 2 = 4 electrons 1 s

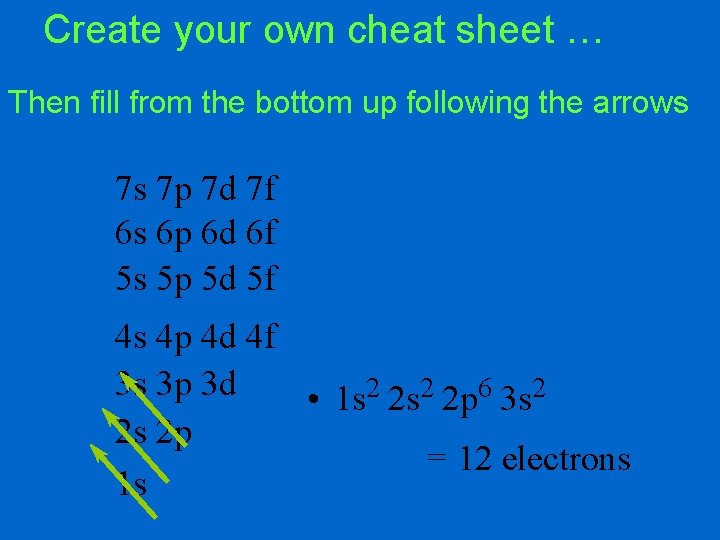
Create your own cheat sheet … Then fill from the bottom up following the arrows 7 s 7 p 7 d 7 f 6 s 6 p 6 d 6 f 5 s 5 p 5 d 5 f 4 s 4 p 4 d 4 f 3 s 3 p 3 d • 1 s 2 2 p 6 3 s 2 2 s 2 p = 12 electrons 1 s

Create your own cheat sheet … Then fill from the bottom up following the arrows 7 s 7 p 7 d 7 f 6 s 6 p 6 d 6 f 5 s 5 p 5 d 5 f 4 s 4 p 4 d 4 f 3 s 3 p 3 d 2 s 2 p 1 s • 2 2 6 2 1 s 2 s 2 p 3 s 3 p 4 s = 20 electrons

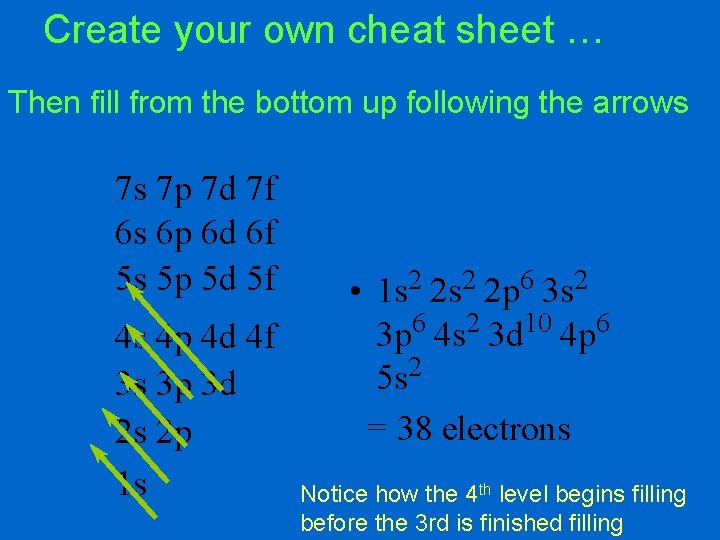
Create your own cheat sheet … Then fill from the bottom up following the arrows 7 s 7 p 7 d 7 f 6 s 6 p 6 d 6 f 5 s 5 p 5 d 5 f 4 s 4 p 4 d 4 f 3 s 3 p 3 d 2 s 2 p 1 s • 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 2 5 s = 38 electrons Notice how the 4 th level begins filling before the 3 rd is finished filling

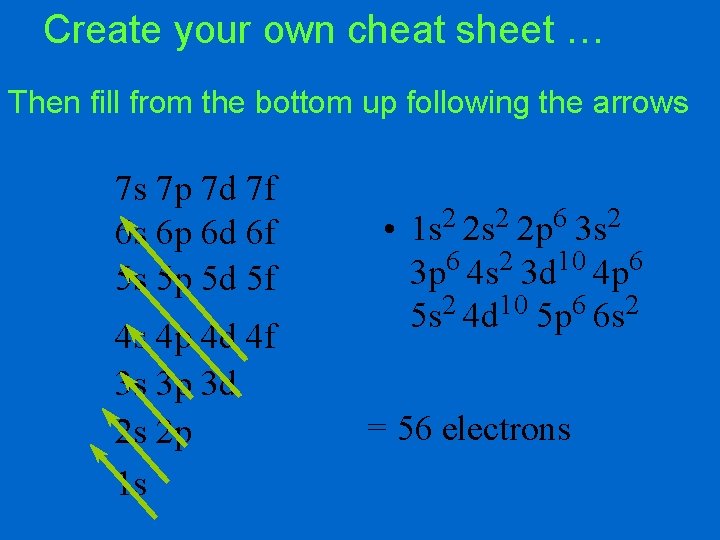
Create your own cheat sheet … Then fill from the bottom up following the arrows 7 s 7 p 7 d 7 f 6 s 6 p 6 d 6 f 5 s 5 p 5 d 5 f 4 s 4 p 4 d 4 f 3 s 3 p 3 d 2 s 2 p 1 s • 2 2 6 2 1 s 2 s 2 p 3 s 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 2 = 56 electrons

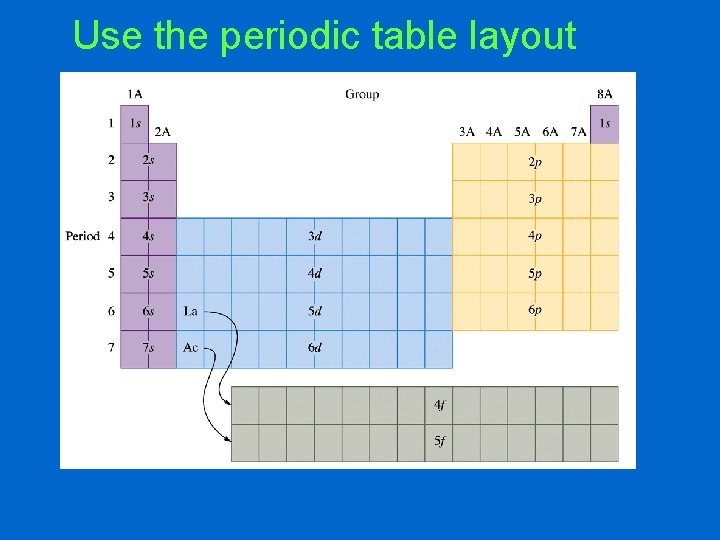
Use the periodic table layout

Use the periodic table layout

More Practice: # e Na 11 Configuration 1 s 22 p 63 s 1 Cl 17 1 s 22 p 63 s 23 p 5 Sb 51 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 3 K 19 1 s 22 p 63 s 23 p 64 s 1

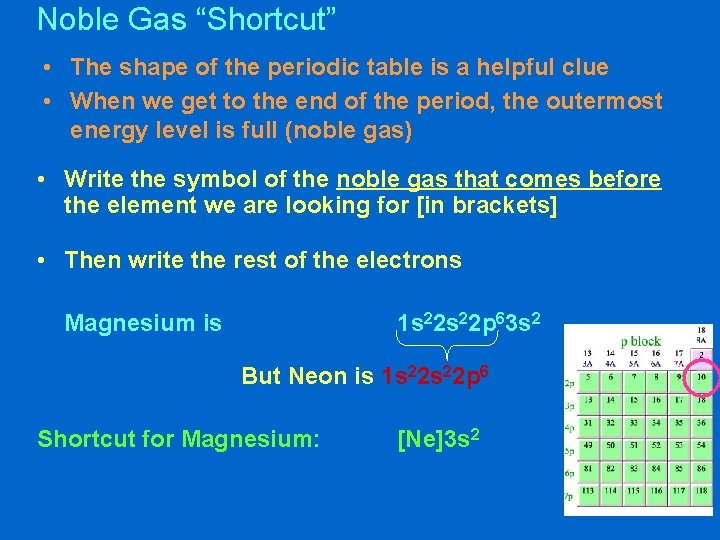
Noble Gas “Shortcut” • The shape of the periodic table is a helpful clue • When we get to the end of the period, the outermost energy level is full (noble gas) • Write the symbol of the noble gas that comes before the element we are looking for [in brackets] • Then write the rest of the electrons Magnesium is 1 s 22 p 63 s 2 But Neon is 1 s 22 p 6 Shortcut for Magnesium: [Ne]3 s 2

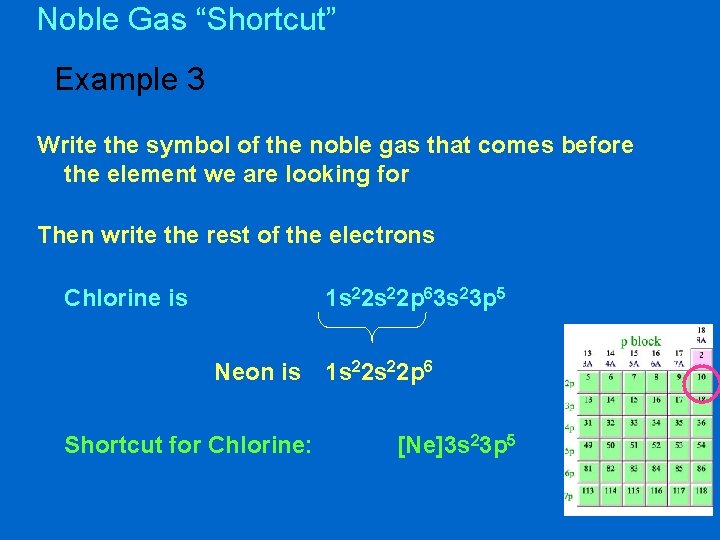
Noble Gas “Shortcut” Example 3 Write the symbol of the noble gas that comes before the element we are looking for Then write the rest of the electrons Chlorine is 1 s 22 p 63 s 23 p 5 Neon is Shortcut for Chlorine: 1 s 22 p 6 [Ne]3 s 23 p 5

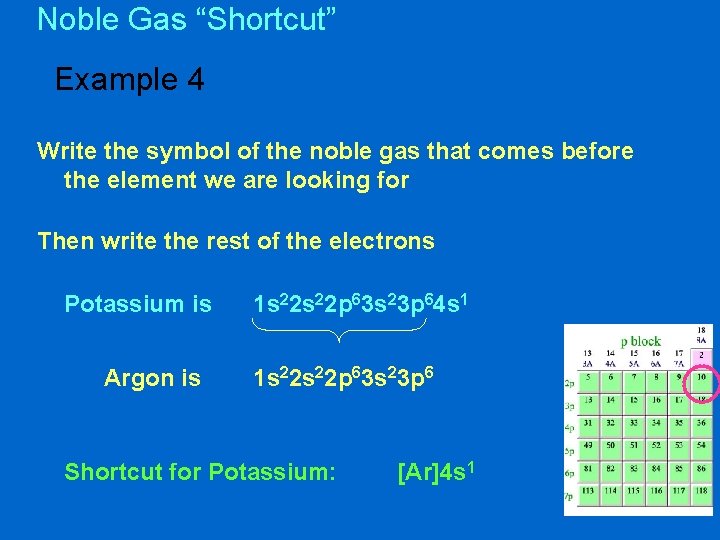
Noble Gas “Shortcut” Example 4 Write the symbol of the noble gas that comes before the element we are looking for Then write the rest of the electrons Potassium is Argon is 1 s 22 p 63 s 23 p 64 s 1 1 s 22 p 63 s 23 p 6 Shortcut for Potassium: [Ar]4 s 1

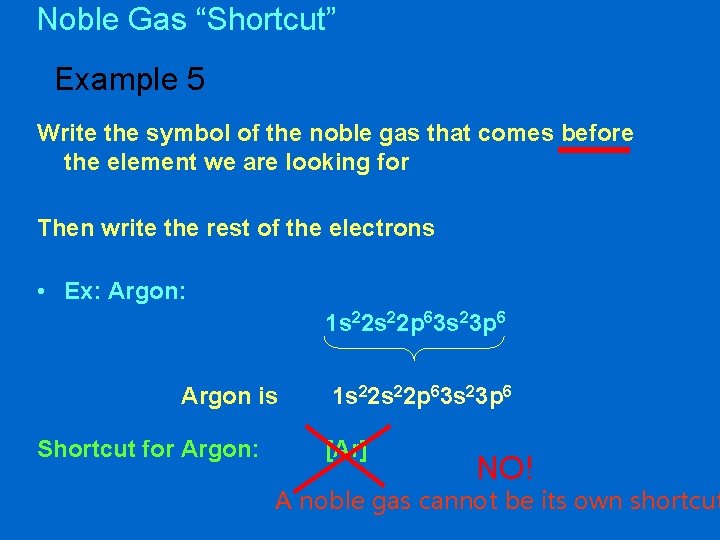
Noble Gas “Shortcut” Example 5 Write the symbol of the noble gas that comes before the element we are looking for Then write the rest of the electrons • Ex: Argon: 1 s 22 p 63 s 23 p 6 Argon is Shortcut for Argon: 1 s 22 p 63 s 23 p 6 [Ar] NO! A noble gas cannot be its own shortcut

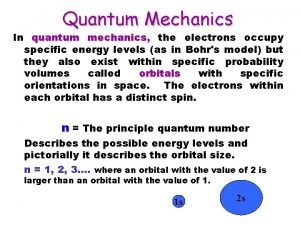
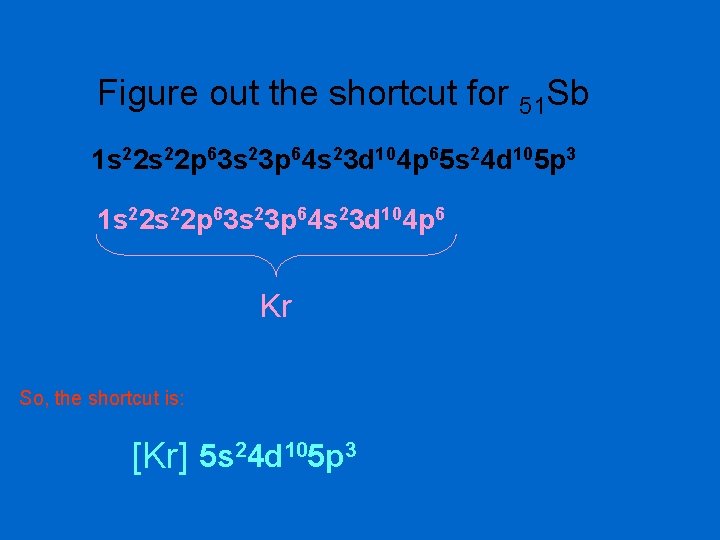
Figure out the shortcut for 51 Sb 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 3 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 Kr So, the shortcut is: [Kr] 5 s 24 d 105 p 3

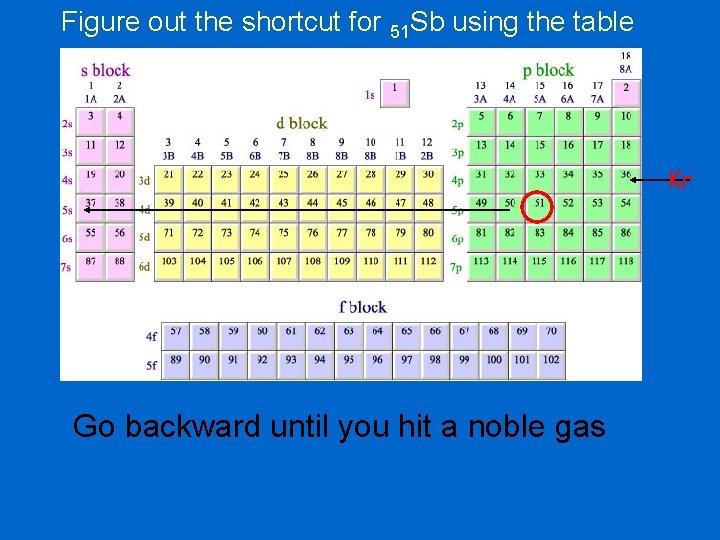
Figure out the shortcut for 51 Sb using the table Kr Go backward until you hit a noble gas
![Figure out the shortcut for 51 Sb using the table Kr 5 s 2 Figure out the shortcut for 51 Sb using the table [Kr] 5 s 2](https://slidetodoc.com/presentation_image_h2/25f10ccb50897f75da13e13f25d72e7a/image-76.jpg)
Figure out the shortcut for 51 Sb using the table [Kr] 5 s 2 4 d 10 5 p 3

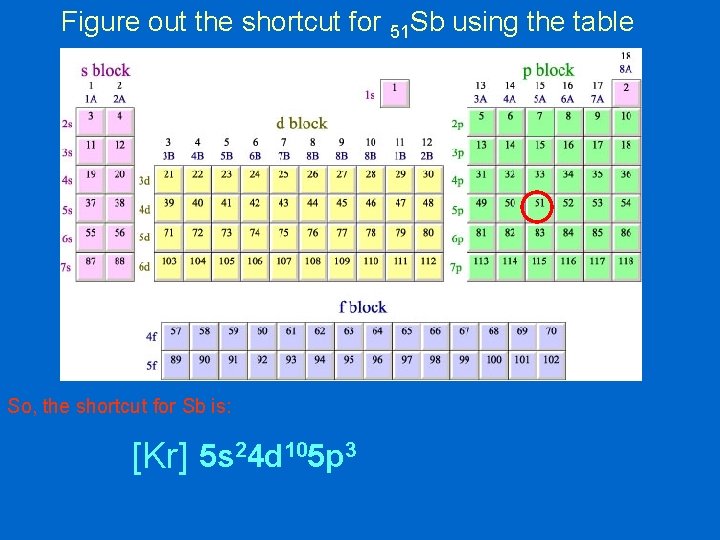
Figure out the shortcut for 51 Sb using the table So, the shortcut for Sb is: [Kr] 5 s 24 d 105 p 3

Write the complete electron configuration and the noble gas shortcut electron configuration for Polonium (Po) Xenon (Xe)

Orbital Diagrams and Electron Configuration ws

 Origin of quantum mechanics
Origin of quantum mechanics Quantum physics vs quantum mechanics
Quantum physics vs quantum mechanics Eigenfunction
Eigenfunction Expectation value of energy in quantum mechanics
Expectation value of energy in quantum mechanics Incident wave equation
Incident wave equation Expectation value of energy in quantum mechanics
Expectation value of energy in quantum mechanics French and taylor quantum mechanics
French and taylor quantum mechanics Quantum mechanics in your face
Quantum mechanics in your face Chemistry
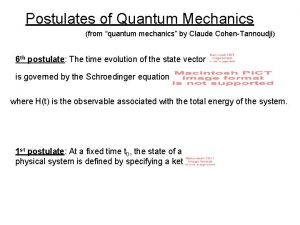
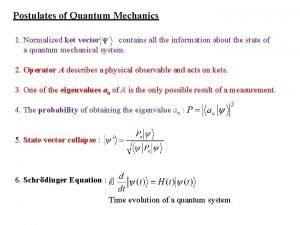
Chemistry Postulates of quantum mechanics
Postulates of quantum mechanics Ket 0 and ket 1
Ket 0 and ket 1 Operators in quantum mechanics
Operators in quantum mechanics Dr susan cartwright
Dr susan cartwright Operators in quantum mechanics
Operators in quantum mechanics Schroendiger
Schroendiger Beta plus decay
Beta plus decay Instantons
Instantons Expectation value in quantum mechanics
Expectation value in quantum mechanics Operators in quantum mechanics
Operators in quantum mechanics Quantum mechanics in three dimensions
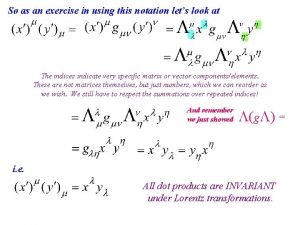
Quantum mechanics in three dimensions The basics of quantum mechanics
The basics of quantum mechanics Schrodinger cat
Schrodinger cat Spin angular momentum formula
Spin angular momentum formula Introduction to quantum statistical mechanics
Introduction to quantum statistical mechanics Quantum game theory
Quantum game theory Commutation relation in quantum mechanics
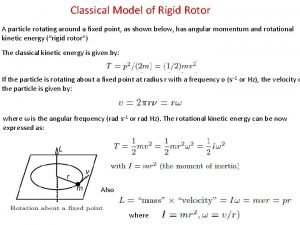
Commutation relation in quantum mechanics 2d rigid rotor
2d rigid rotor Review of quantum mechanics
Review of quantum mechanics Griffiths
Griffiths Transfer matrix quantum mechanics
Transfer matrix quantum mechanics Littlejohn quantum mechanics
Littlejohn quantum mechanics Quantum mechanics powerpoint
Quantum mechanics powerpoint Central potential in quantum mechanics
Central potential in quantum mechanics Quantum mechanics
Quantum mechanics Bohr magneton class 12
Bohr magneton class 12 Postulates of quantum mechanics
Postulates of quantum mechanics Operator in quantum mechanics
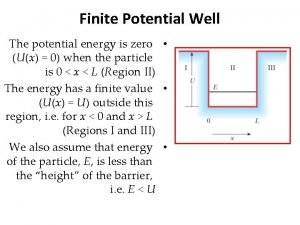
Operator in quantum mechanics Potential energy well
Potential energy well Copenhagen interpretation
Copenhagen interpretation Ahmaths
Ahmaths Understanding standards advanced higher english
Understanding standards advanced higher english Sublevel d
Sublevel d Quantum chemistry
Quantum chemistry Unit 6 review questions
Unit 6 review questions Shop safety color coding system
Shop safety color coding system Further mechanics 1 unit test 1 momentum and impulse
Further mechanics 1 unit test 1 momentum and impulse For a broad base of support feet should be
For a broad base of support feet should be Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Salters advanced chemistry
Salters advanced chemistry Advanced medicinal chemistry
Advanced medicinal chemistry External forms of social control criminology
External forms of social control criminology Criminology unit 4 revision
Criminology unit 4 revision Advanced higher biology unit 1
Advanced higher biology unit 1 Advanced higher biology unit 3 questions
Advanced higher biology unit 3 questions Advanced higher biology unit 2
Advanced higher biology unit 2 Sex determination and sex linkage
Sex determination and sex linkage Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Unit 9 lesson 4
Unit 9 lesson 4 Ap chem unit 7
Ap chem unit 7 Chemistry unit 7 molarity
Chemistry unit 7 molarity Q=mc∆t
Q=mc∆t Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Units of concentration
Units of concentration Wjec chemistry unit 2
Wjec chemistry unit 2 Unit 8 ap chemistry
Unit 8 ap chemistry Chemistry grade 11 unit 4 chemical kinetics
Chemistry grade 11 unit 4 chemical kinetics Chemistry unit 3 test answer key
Chemistry unit 3 test answer key Chemistry review answer key
Chemistry review answer key Chemistry grade 10 unit 4
Chemistry grade 10 unit 4 Unit 6 chemistry review
Unit 6 chemistry review Ap chemistry unit 9 notes
Ap chemistry unit 9 notes Higher chemistry unit 3 notes
Higher chemistry unit 3 notes Wjec chemistry unit 2
Wjec chemistry unit 2 Chemistry unit 6 sticky tape post lab
Chemistry unit 6 sticky tape post lab Living by chemistry unit 2 smells answers
Living by chemistry unit 2 smells answers Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Ap chemistry unit 3
Ap chemistry unit 3 Ap chemistry unit 2
Ap chemistry unit 2 What is the answer
What is the answer Three characteristics of metals
Three characteristics of metals