Physics 102 Lecture 22 Quantum Mechanics Blackbody Radiation

Physics 102: Lecture 22 Quantum Mechanics: Blackbody Radiation, Photoelectric Effect, Wave-Particle Duality Physics 102: Lecture 22, Slide 1

Recap. • Interference: Coherent waves – Full wavelength difference = Constructive – ½ wavelength difference = Destructive opposite! • Multiple Slits – Constructive d sin(q) = m l (m=1, 2, 3…) – Destructive d sin(q) = (m + 1/2) l 2 slit only – More slits = brighter max, darker mins • Huygens’ Principle: Each point on wave front acts as coherent source and can interfere. (see Lab 8) • Single Slit: – Destructive: w sin(q) = m l (m=1, 2, 3…) – Resolution: Max from 1 at Min from 2 Physics 102: Lecture 22, Slide 2

State of Late 19 th Century Physics • Two great theories – Newton’s laws of mechanics, including gravity – Maxwell’s theory of electricity & magnetism, including propagation of electromagnetic waves • But…some unsettling experimental results calls into question these theories – Einstein and relativity – The quantum revolution Physics 102: Lecture 22, Slide 3

Physics 102: Lecture 22, Slide 4

Quantum Mechanics! • At very small sizes the world is VERY different! – Energy is discrete, not continuous. – Everything is probability; nothing is for certain. – Particles often seem to be in two places at same time. – Looking at something changes how it behaves. • If you aren’t confused by the end of this lecture, you weren’t paying attention! Physics 102: Lecture 22, Slide 5
Three Early Indications of Problems with Classical Physics • Blackbody radiation • Photoelectric effect • Wave-particle duality Physics 102: Lecture 22, Slide 6

Blackbody Radiation Hot objects glow (toaster coils, light bulbs, the sun). As the temperature increases the color shifts from Red to Blue. The classical physics prediction was completely wrong! (It said that an infinite amount of energy should be radiated by an object at finite temperature. ) Physics 102: Lecture 22, Slide 7

Physics 102: Lecture 22, Slide 8
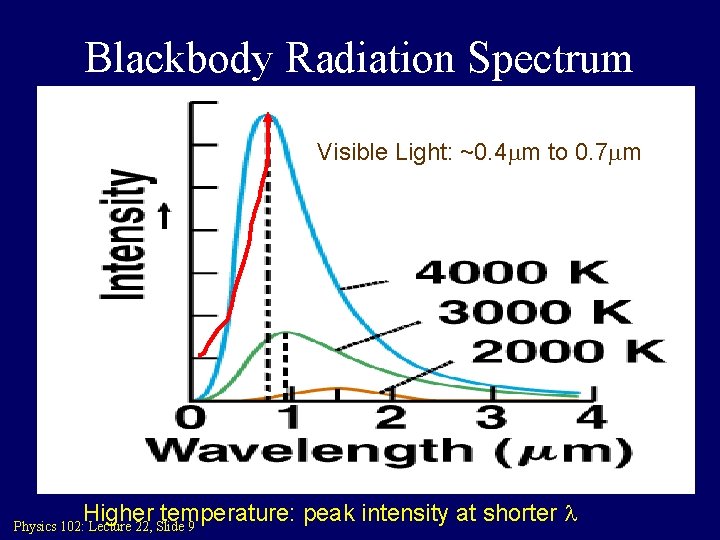
Blackbody Radiation Spectrum Visible Light: ~0. 4 mm to 0. 7 mm Higher temperature: peak intensity at shorter l Physics 102: Lecture 22, Slide 9

Blackbody Radiation: First evidence for Q. M. Max Planck found he could explain these curves if he assumed that electromagnetic energy was radiated in discrete chunks, rather than continuously. The “quanta” of electromagnetic energy is called the photon. Energy carried by a single photon is E = hf = hc/l Planck’s constant: h = 6. 626 X 10 -34 Joule sec Physics 102: Lecture 22, Slide 10

Preflights 22. 1, 22. 3 A series of light bulbs are colored red, yellow, and blue. Which bulb emits photons with the most energy? Blue! Lowest wavelength is highest energy. The least energy? E = hf = hc/l Red! Highest wavelength is lowest energy. Which is hotter? (1) stove burner glowing red (2) stove burner glowing orange Physics 102: Lecture 22, Slide 11

Physics 102: Lecture 22, Slide 12

ACT: Photon A red and green laser are each rated at 2. 5 m. W. Which one produces more photons/second? 1) Red Physics 102: Lecture 22, Slide 13 2) Green 3) Same

Nobel Trivia For which work did Einstein receive the Nobel Prize? 1) Special Relativity E=mc 2 2) General Relativity Gravity bends Light 3) Photoelectric Effect Photons 4) Einstein didn’t receive a Nobel prize. Physics 102: Lecture 22, Slide 14
Photoelectric Effect • Light shining on a metal can “knock” electrons out of atoms. • Light must provide energy to overcome Coulomb attraction of electron to nucleus • Light Intensity gives power/area (i. e. Watts/m 2) – Recall: Power = Energy/time (i. e. Joules/sec. ) Physics 102: Lecture 22, Slide 15

Physics 102: Lecture 22, Slide 16

Photoelectric Effect: Light Intensity • What happens to the rate electrons are emitted when increase the brightness? • What happens to max kinetic energy when increase brightness? Physics 102: Lecture 22, Slide 17
Photoelectric Effect: Light Frequency • What happens to rate electrons are emitted when increase the frequency of the light? • What happens to max kinetic energy when increase the frequency of the light? Physics 102: Lecture 22, Slide 18
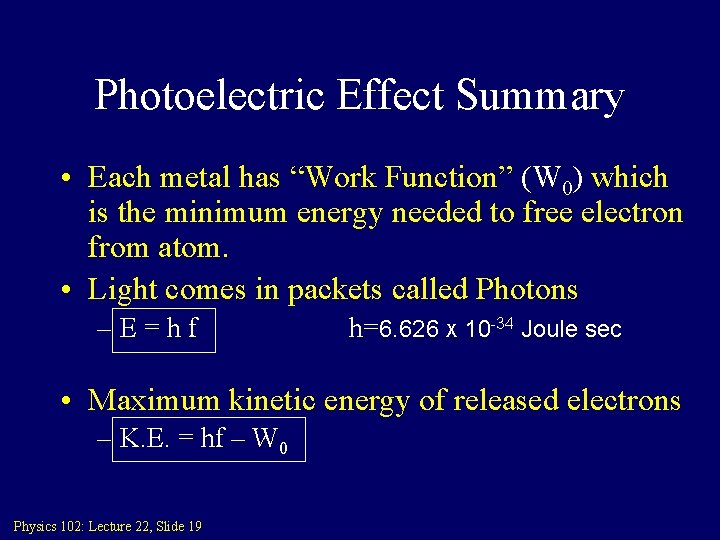
Photoelectric Effect Summary • Each metal has “Work Function” (W 0) which is the minimum energy needed to free electron from atom. • Light comes in packets called Photons –E=hf h=6. 626 X 10 -34 Joule sec • Maximum kinetic energy of released electrons – K. E. = hf – W 0 Physics 102: Lecture 22, Slide 19

Physics 102: Lecture 22, Slide 20
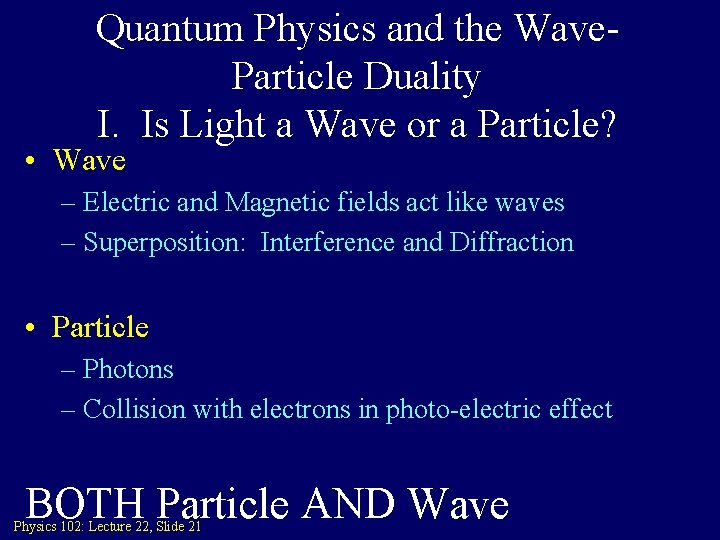
Quantum Physics and the Wave. Particle Duality I. Is Light a Wave or a Particle? • Wave – Electric and Magnetic fields act like waves – Superposition: Interference and Diffraction • Particle – Photons – Collision with electrons in photo-electric effect BOTH Particle AND Wave Physics 102: Lecture 22, Slide 21
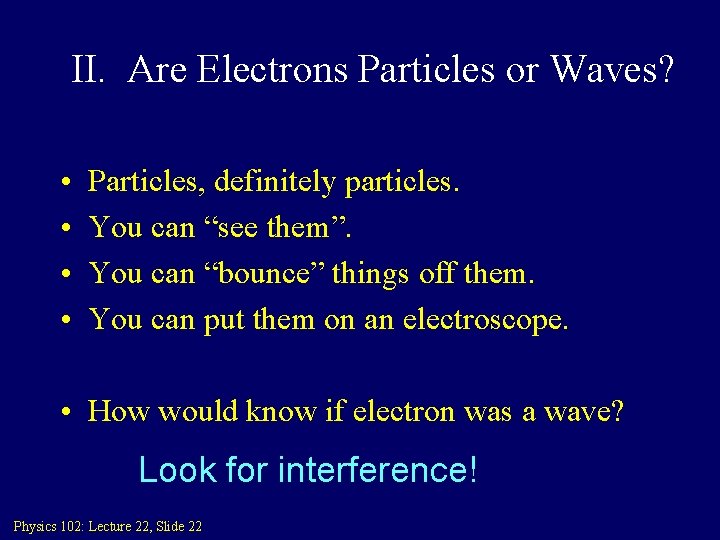
II. Are Electrons Particles or Waves? • • Particles, definitely particles. You can “see them”. You can “bounce” things off them. You can put them on an electroscope. • How would know if electron was a wave? Look for interference! Physics 102: Lecture 22, Slide 22
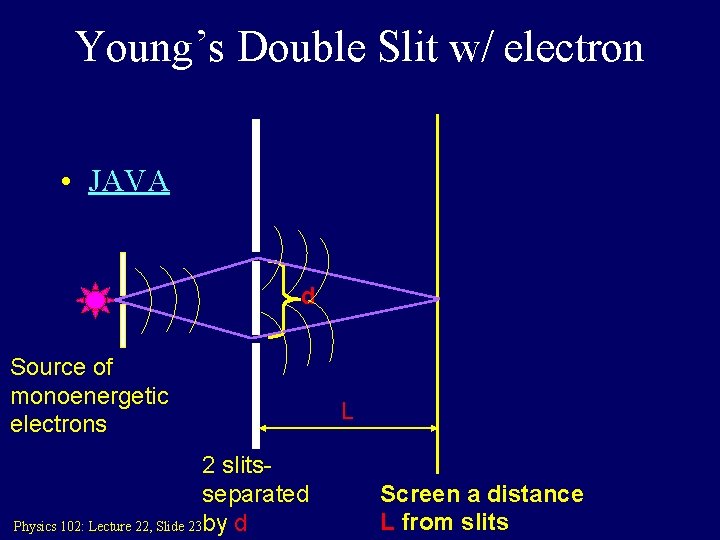
Young’s Double Slit w/ electron • JAVA d Source of monoenergetic electrons 2 slitsseparated Physics 102: Lecture 22, Slide 23 by d L Screen a distance L from slits

Physics 102: Lecture 22, Slide 24
Electrons are Waves? • Electrons produce interference pattern just like light waves. – Need electrons to go through both slits. – What if we send 1 electron at a time? – Does a single electron go through both slits? Physics 102: Lecture 22, Slide 25

ACT: Electrons are Particles • If we shine a bright light, we can ‘see’ which hole the electron goes through. (1) Both Slits Physics 102: Lecture 22, Slide 26 (2) Only 1 Slit

Electrons are Particles and Waves! • Depending on the experiment electron can behave like – wave (interference) – particle (localized mass and charge) • If we don’t look, electron goes through both slits. If we do look it chooses 1. I’m not kidding it’s true! Physics 102: Lecture 22, Slide 27 46

Physics 102: Lecture 22, Slide 28

Schrödinger's Cat • Place cat in box with some poison. If we don’t look at the cat it will be both dead and alive! Poison Physics 102: Lecture 22, Slide 29

More Nobel Prizes! • 1906 J. J. Thompson – Showing cathode rays are particles (electrons). • 1937 G. P. Thompson (JJ’s son) – Showed electrons are really waves. • Both were right! Physics 102: Lecture 22, Slide 30

Quantum Summary • Particles act as waves and waves act as particles • Physics is NOT deterministic • Observations affect the experiment – (coming soon!) Physics 102: Lecture 22, Slide 31

Physics 102: Lecture 22, Slide 32
- Slides: 32