AP CHEMISTRY UNIT 2 ATOMS MOLECULES AND IONS









- Slides: 17
AP CHEMISTRY UNIT 2 – ATOMS, MOLECULES, AND, IONS Day 3 – Finish Analysis of Food Dyes and Mass Spectroscopy

WARM UP SIT WITH LAB PARTNERS TAKE OUT: LAB NOTEBOOK MAKE: “ANALYSIS” SECTION BELOW DATA SECTION FIND: YOUR GRAPHS IN BLUE BIN FOLD, CUT, AND TAPE GRAPHS: INTO ANALYSIS SECTION ON YOUR GRAPHS: FIND EQUATIONS FOR ABSORBANCE AND %TRANSMITTANCE WRITE: EQUATIONS IN ANALYSIS SECTION TIME: 6 MINUTES

AP CHEMISTRY AGENDA • FINISH ANALYSIS OF FOOD DYES LAB • UNIT 2 NOTES (MASS SPECTROSCOPY) • GUIDED INQUIRY ASSIGNMENT NEXT CLASSES: • LAB NOTEBOOK DUE TUESDAY WITH ANALYSIS, CONCLUSION, SOURCES OF ERROR, AND POST-LAB QUESTIONS


FINISH ANALYSIS OF FOOD DYES FIND: LAB HANDOUT “GUIDED INQUIRY” SECTION READ: 1, 2, 4 TALK: WITH LAB PARTNERS TO DETERMINE AN APPROPRIATE PROCEDURE TO DETERMINE CONCENTRATION OF BLUE DYE TIME: 6 MINUTES

FINISH ANALYSIS OF FOOD DYES YOU WILL: OBSERVE HOW MS. MYRIAH OBTAINS THE ABSORBANCE OF THE GLACIER FREEZE GATORADE THEN: RECORD THE ABSORBANCE IN YOUR DATA TABLE IN ANALYSIS SECTION: CALCULATE THE NUMBER OF MILLIGRAMS OF THE GLACIER FREEZE GATORADE TIME: 6 MINUTES WHEN DONE: BE READY TO SHARE

REMEMBER! COMPLETE: THE FOLLOWING SECTIONS BY TUESDAY • ANALYSIS SECTION • CONCLUSION • SOURCES OF ERROR • POST LAB QUESTIONS IF YOU NEED HELP: REFER TO LAB COMPONENT FOR LAB NOTEBOOK, CLASS WEBSITE, THE INTERNET, OR MESSAGE ME (INCLUDE PICTURES IF

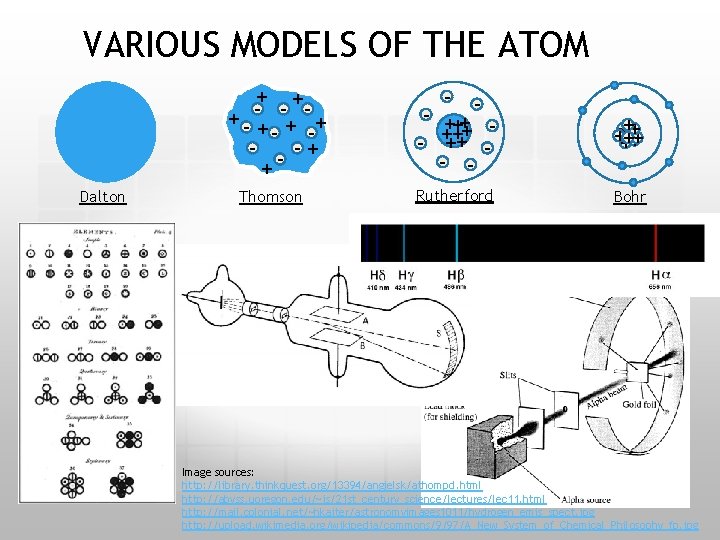
VARIOUS MODELS OF THE ATOM + + - - ++ - + -+ -+ + Dalton Thomson - - - + ++ ++ + - ++ - - + +++++ ++ Rutherford Bohr Image sources: http: //library. thinkquest. org/13394/angielsk/athompd. html http: //abyss. uoregon. edu/~js/21 st_century_science/lectures/lec 11. html http: //mail. colonial. net/~hkaiter/astronomyimages 1011/hydrogen_emis_spect. jpg http: //upload. wikimedia. org/wikipedia/commons/9/97/A_New_System_of_Chemical_Philosophy_fp. jpg

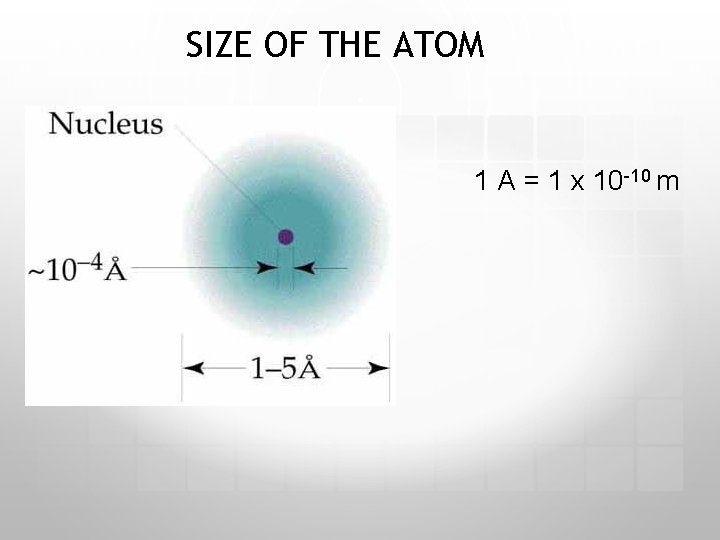
SIZE OF THE ATOM 1 A = 1 x 10 -10 m

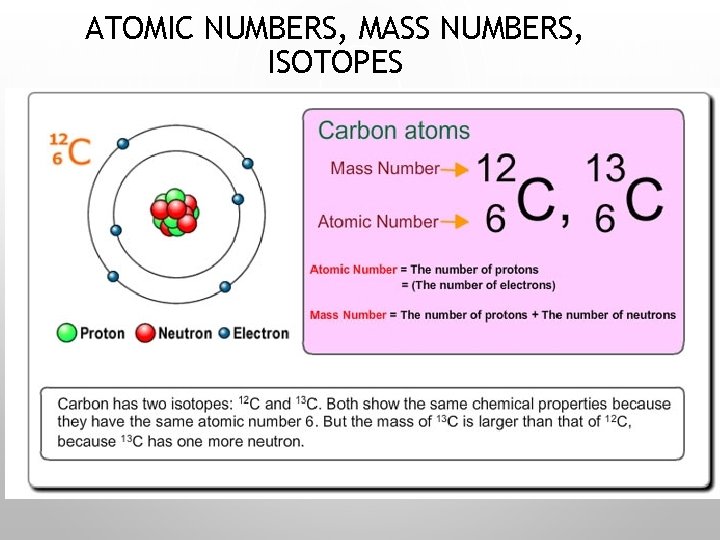
ATOMIC NUMBERS, MASS NUMBERS, ISOTOPES


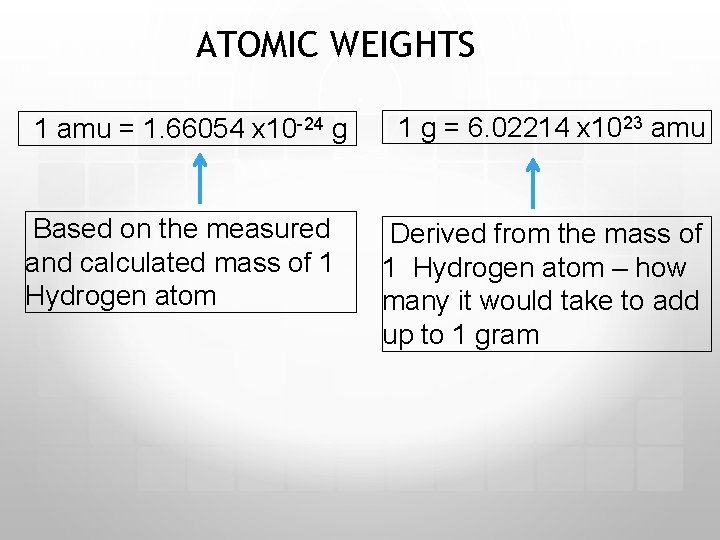
ATOMIC WEIGHTS 1 amu = 1. 66054 x 10 -24 g Based on the measured and calculated mass of 1 Hydrogen atom 1 g = 6. 02214 x 1023 amu Derived from the mass of 1 Hydrogen atom – how many it would take to add up to 1 gram

MASS SPECTROSCOPY VIDEO ON NOTEBOOK PAGE: Draw and write notes about Mass Spectroscopy from video MAKE SURE TO: Write Flow Chart

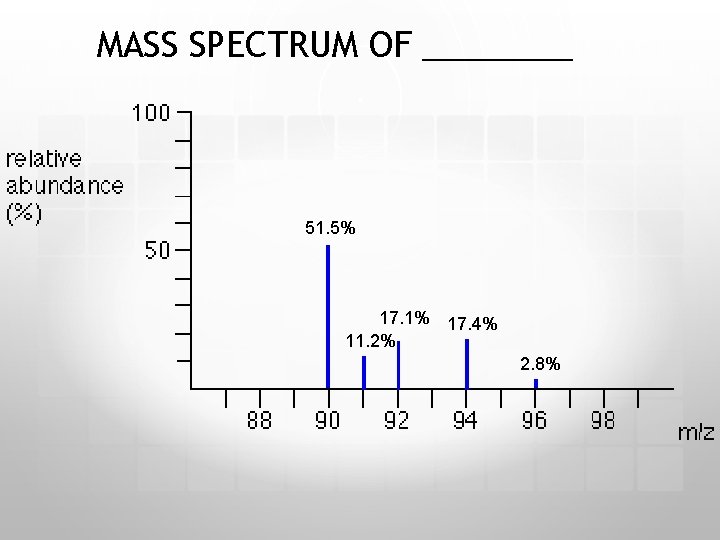
MASS SPECTRUM OF ____ 51. 5% 17. 1% 17. 4% 11. 2% 2. 8%


GUIDED INQUIRY: ATOMIC NUMBER AND MASS WITH THIS GROUP: WORK ON ATOMIC NUMBER AND ATOMIC MASS GUIDED INQUIRY • CTQ = CRITICAL THINKING QUESTIONS TIME: UNTIL END OF CLASS WHEN DONE: HAVE A GREAT WEEKEND AND SEND A MESSAGE IF YOU HAVE A

WHAT WILL YOU NEED TO KNOW FOR UNIT 2 WHAT DO YOU NEED TO KNOW? • CALCULATE ISOTOPES (GUIDED INQUIRY) • MASS SPECTROSCOPY • DALTON’S LAWS • EMPIRICAL FORMULAS • ELECTRON CONFIGURATIONS • HOW TO USE PLANCK’S EQUATION • HOW TO USE BEER’S LAW
 Ion chapter 11
Ion chapter 11 Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules Collision theory states that
Collision theory states that Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Positive ions and negative ions table
Positive ions and negative ions table Relationship between atoms and molecules
Relationship between atoms and molecules Atoms elements molecules and compounds worksheet
Atoms elements molecules and compounds worksheet 3bacl2 counting atoms
3bacl2 counting atoms Interacting molecules or ions
Interacting molecules or ions Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Positive ions are atoms that have
Positive ions are atoms that have Atoms or ions are considered isoelectronic if
Atoms or ions are considered isoelectronic if Atoms combine to form
Atoms combine to form Periodic table of elements regents
Periodic table of elements regents