Unit 9 Lesson 4 What Are Some Changes











- Slides: 15

Unit 9 Lesson 4 What Are Some Changes to Matter? Copyright © Houghton Mifflin Harcourt Publishing Company

Unit 9 Lesson 4 What Are Some Changes to Matter? Physical Changes • A change to matter can either be physical or chemical. Copyright © Houghton Mifflin Harcourt Publishing Company

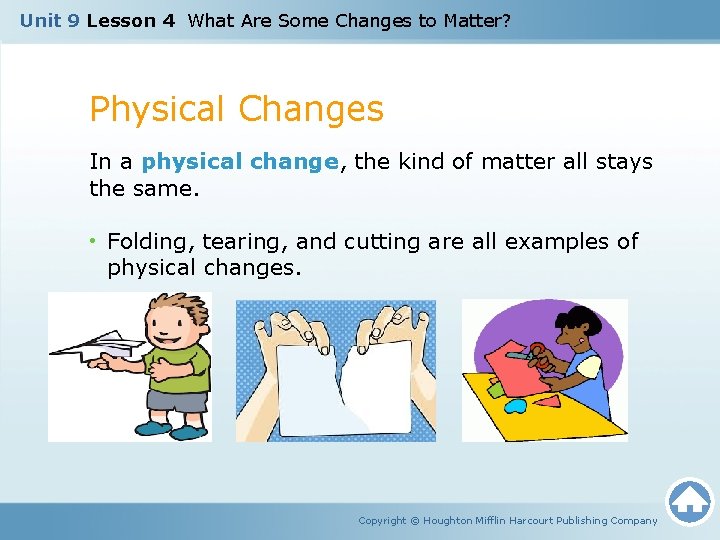
Unit 9 Lesson 4 What Are Some Changes to Matter? Physical Changes In a physical change, the kind of matter all stays the same. • Folding, tearing, and cutting are all examples of physical changes. Copyright © Houghton Mifflin Harcourt Publishing Company

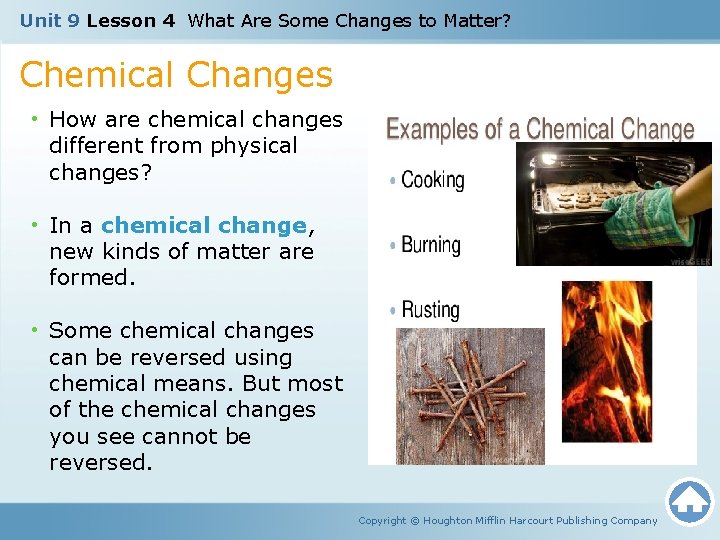
Unit 9 Lesson 4 What Are Some Changes to Matter? Chemical Changes • How are chemical changes different from physical changes? • In a chemical change, new kinds of matter are formed. • Some chemical changes can be reversed using chemical means. But most of the chemical changes you see cannot be reversed. Copyright © Houghton Mifflin Harcourt Publishing Company

Unit 9 Lesson 4 What Are Some Changes to Matter? Chemical Changes • Chemical changes happen all around you. • Chemical changes can happen in your body. • Suppose you eat a pear. As your body digests the pear, chemical changes break it up into simpler pieces that your body can use. Copyright © Houghton Mifflin Harcourt Publishing Company

Unit 9 Lesson 4 What Are Some Changes to Matter? Physical changes Vs. Chemical Changes Copyright © Houghton Mifflin Harcourt Publishing Company

Unit 9 Lesson 4 What Are Some Changes to Matter? Physical change Vs. Chemical Changes Copyright © Houghton Mifflin Harcourt Publishing Company

Unit 9 Lesson 4 What Are Some Changes to Matter? Physical change Vs. Chemical Changes Copyright © Houghton Mifflin Harcourt Publishing Company

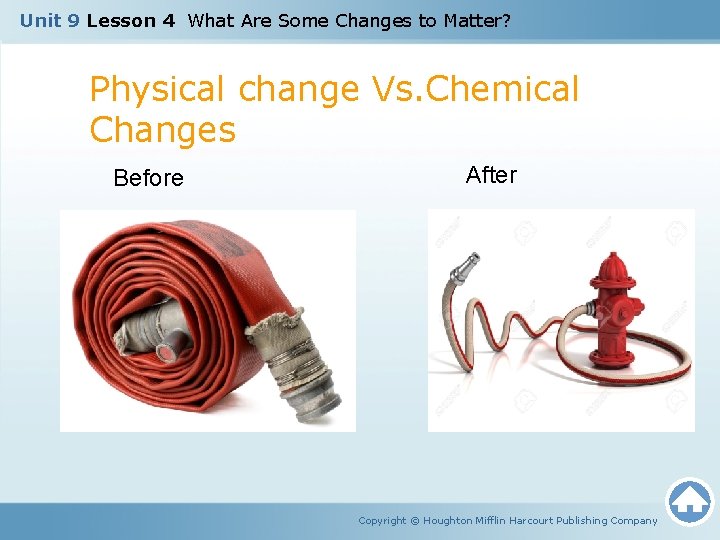
Unit 9 Lesson 4 What Are Some Changes to Matter? Physical change Vs. Chemical Changes Before After Copyright © Houghton Mifflin Harcourt Publishing Company

Unit 9 Lesson 4 What Are Some Changes to Matter? Mixtures and Solutions • A mixture is two or more substances that are combined without changing any of them. • Because no new matter is formed, making a mixture is a physical change. Copyright © Houghton Mifflin Harcourt Publishing Company

Unit 9 Lesson 4 What Are Some Changes to Matter? Mixtures and Solutions • A solution is a mixture in which all the substances are evenly mixed. • To make a solution, you completely mix, or dissolve, one substance in another. Copyright © Houghton Mifflin Harcourt Publishing Company

Unit 9 Lesson 4 What Are Some Changes to Matter? Mixtures and Solutions • All solutions are mixtures. • Lemonade is a mixture of water, sugar, and lemon juice. • Lemonade is also a solution because the sugar and lemon juice have dissolved in the water. Copyright © Houghton Mifflin Harcourt Publishing Company

Unit 9 Lesson 4 What Are Some Changes to Matter? Properties Matter! • Because making a mixture is a physical change, you can separate a mixture using the physical properties of its parts. • Floating is a physical property. You can use it to separate some mixtures. Just add water, and scoop off the objects that float. • Evaporation can separate mixtures in which solids have been dissolved in liquids. As the liquid evaporates, the solids are left behind. Copyright © Houghton Mifflin Harcourt Publishing Company

Unit 9 Lesson 4 What Are Some Changes to Matter? Properties Matter! • You can also use tools such as sieves and magnets to separate mixtures. • Matter that is smaller than the holes in a sieve passes through the sieve. Matter that is larger than the holes stays on top of the sieve. • A magnet picks up matter that contains iron. A giant magnet can separate iron from plastic and other materials in a junkyard. Copyright © Houghton Mifflin Harcourt Publishing Company

Unit 9 Lesson 4 What Are Some Changes to Matter? Making Bagels • A kitchen is filled with changes in matter as bagels are made. • Hands and kitchen tools cause physical changes. • Yeast and heat cause chemical changes. Copyright © Houghton Mifflin Harcourt Publishing Company