Atomic Theory Quantum Mechanics Quantum Mechanics The ol

















![Core Notation Give the electron configuration for Titanium Core Notation for Ti: [Ar] 4 Core Notation Give the electron configuration for Titanium Core Notation for Ti: [Ar] 4](https://slidetodoc.com/presentation_image_h2/144b48fcaeb6939f5dd5379cbabd7df4/image-37.jpg)










- Slides: 48

Atomic Theory Quantum Mechanics

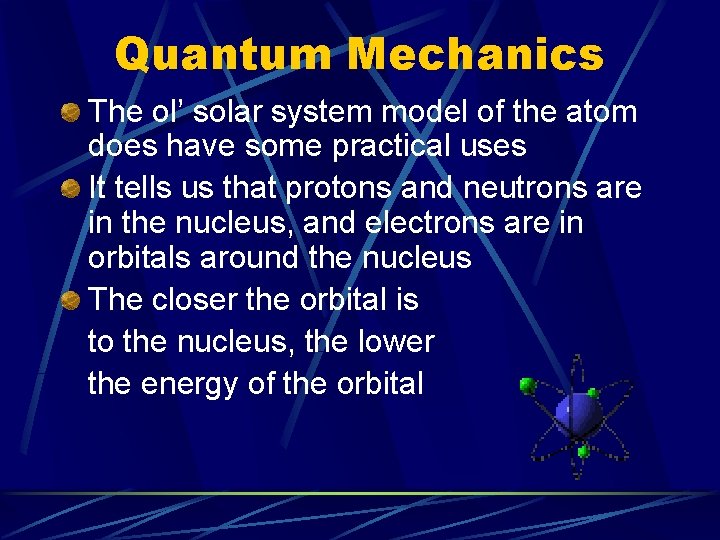
Quantum Mechanics The ol’ solar system model of the atom does have some practical uses It tells us that protons and neutrons are in the nucleus, and electrons are in orbitals around the nucleus The closer the orbital is to the nucleus, the lower the energy of the orbital

But… Electrons do not travel in specific pathways in the orbital as shown in the previous picture Orbitals are not ‘trails’ around the nucleus This model went out with Model-T Fords and Al Capone …just chillin’

The New Look Atom Electrons are in orbitals, but orbitals are clouds of probability that show where an electron could be For example, suppose you go to a school dance. Your mom knows that there is a large probability that you are in the school gym, but cannot pinpoint where in the gym at any one time

Orbitals have different sizes and shapes, depending on their energy level Every orbital can accommodate up to two electrons – each electron spins in a different direction

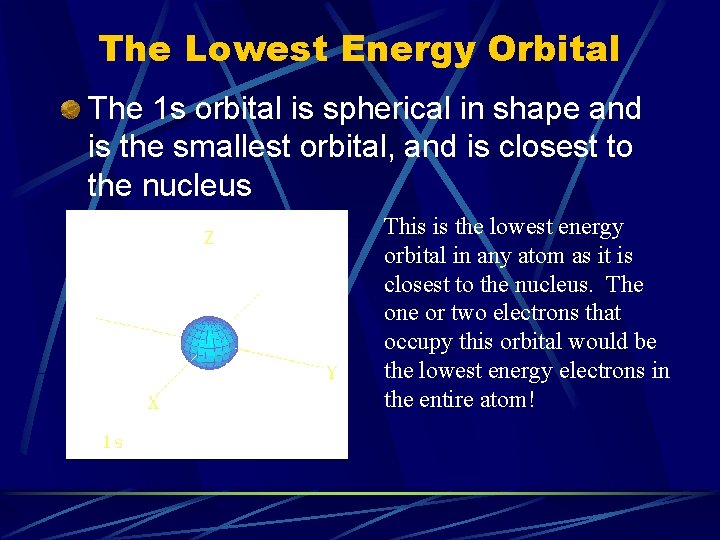
The Lowest Energy Orbital The 1 s orbital is spherical in shape and is the smallest orbital, and is closest to the nucleus This is the lowest energy orbital in any atom as it is closest to the nucleus. The one or two electrons that occupy this orbital would be the lowest energy electrons in the entire atom!

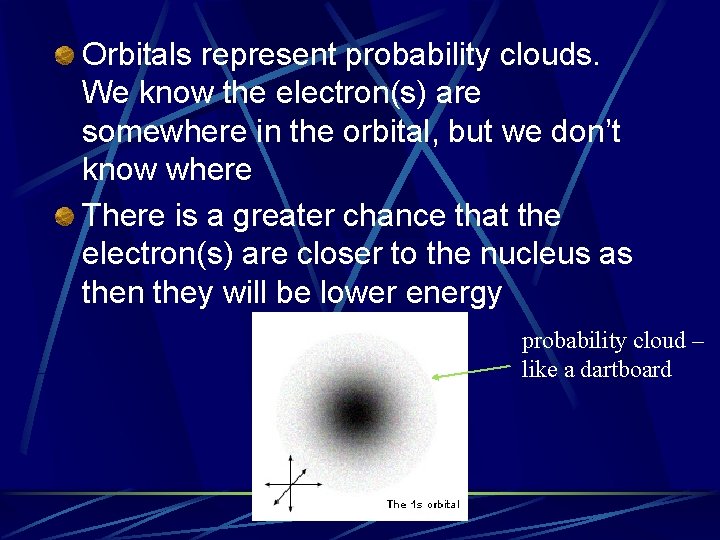
Orbitals represent probability clouds. We know the electron(s) are somewhere in the orbital, but we don’t know where There is a greater chance that the electron(s) are closer to the nucleus as then they will be lower energy probability cloud – like a dartboard

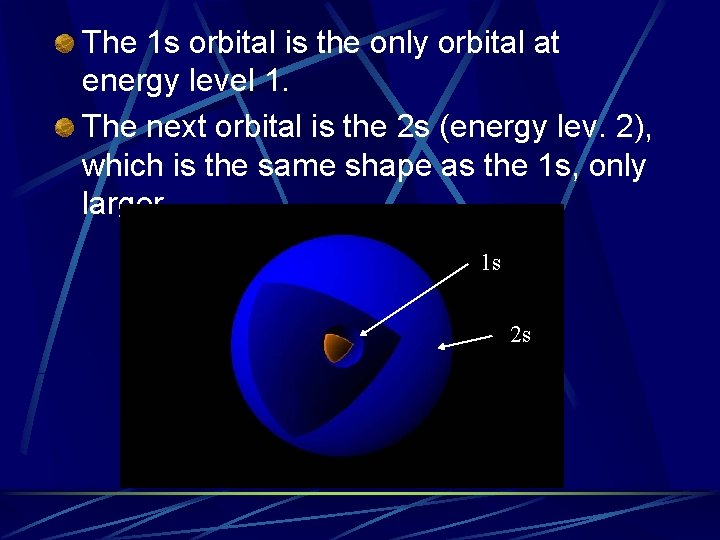
The 1 s orbital is the only orbital at energy level 1. The next orbital is the 2 s (energy lev. 2), which is the same shape as the 1 s, only larger 1 s 2 s

Hydrogen How many electrons does hydrogen have? 1 In its stable state, the hydrogen electron is in the 1 s (lowest energy) orbital. This is called the ‘ground state’ If the electron becomes excited, it can be promoted to the 2 s orbital (excited state), where it will eventually emit the excess energy and return to the 1 s level

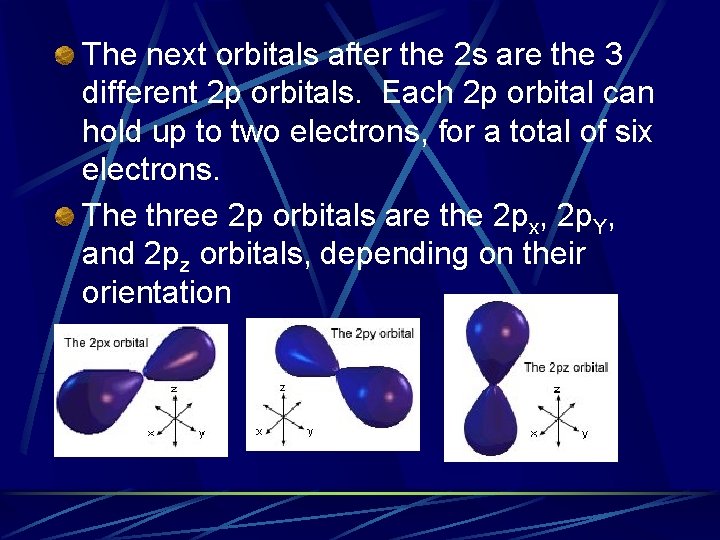
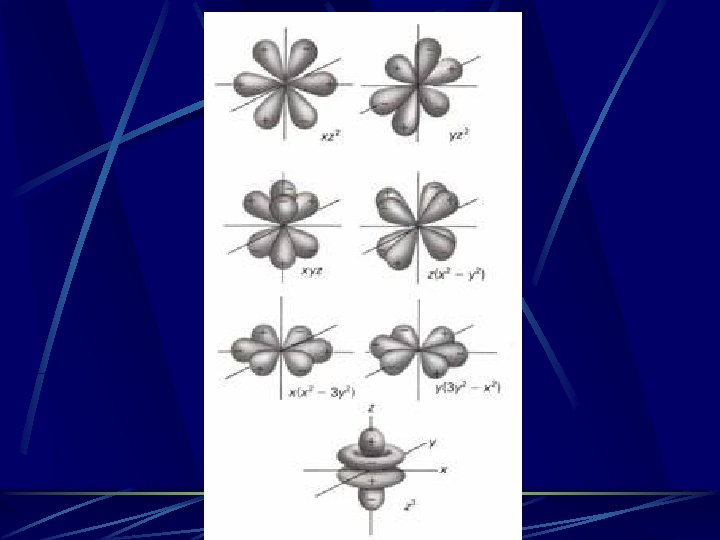
The next orbitals after the 2 s are the 3 different 2 p orbitals. Each 2 p orbital can hold up to two electrons, for a total of six electrons. The three 2 p orbitals are the 2 px, 2 p. Y, and 2 pz orbitals, depending on their orientation

The 2 p Orbs all together Remember – these are probability clouds that represent where the electron(s) could be!


Think back to Science 10… How did the electron shells go again? 2, 8, 8, 18, 32… How many orbitals in energy level 1 in our new model…how many electrons? Only 1 s, therefore 2 electrons In the second energy level? 2 s, 2 px, 2 p. Y, 2 pz, therefore 8 electrons Notice a trend? – Our energy levels are the same as the ‘shells’ you learned about before

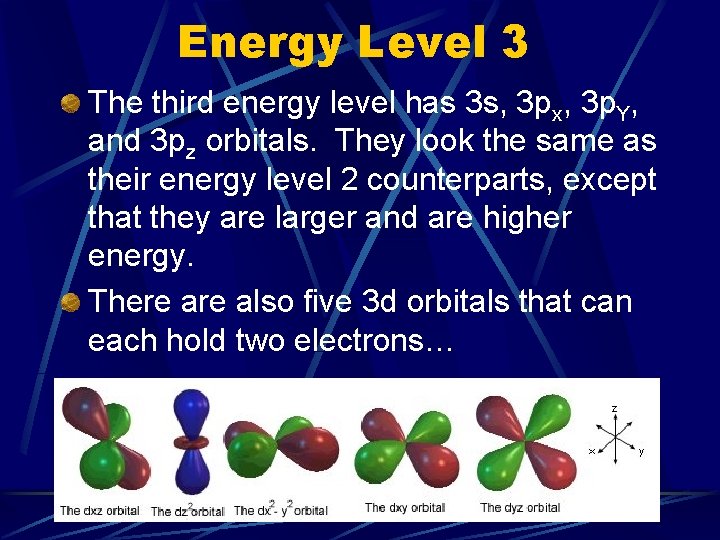
Energy Level 3 The third energy level has 3 s, 3 px, 3 p. Y, and 3 pz orbitals. They look the same as their energy level 2 counterparts, except that they are larger and are higher energy. There also five 3 d orbitals that can each hold two electrons…

Here’s how they look combined… How the Mars Rover landed in 1997…

Energy Level 4 The 4 th energy level has a 4 s orbital, three 4 p orbitals, five 4 d orbitals, and seven 4 f orbitals. The s, p, and d orbitals look like the level 3 orbitals except they are larger and higher energy The seven 4 f orbitals look like this…


Energy Levels 5, 6, 7 Levels 5, 6, and 7 all have one s orbital, three p orbitals, five d orbitals, and seven f orbitals respectively As the level number increases, both the size and energy of the orbitals increase

Electron Configurations Every atom or ion has an electron configuration - a map of where the electrons are We show the electrons in the ground state – the lowest energy arrangement Hydrogen only has one electron – what orbital would it be in? You’re right!! The 1 s orbital

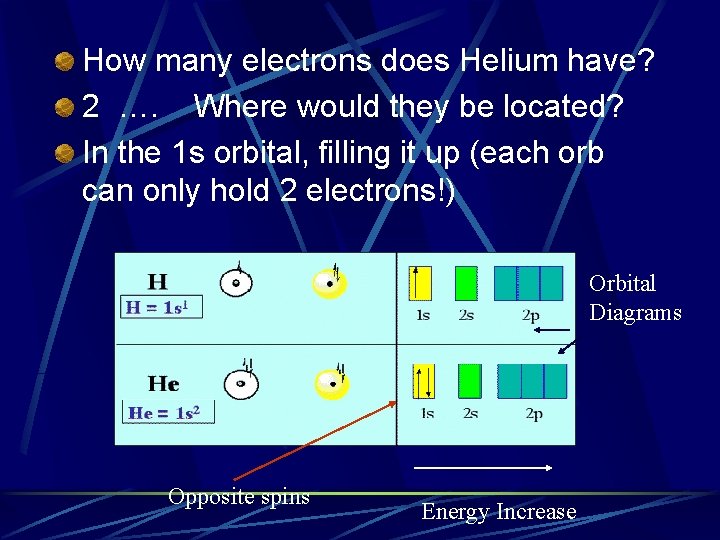
How many electrons does Helium have? 2 …. Where would they be located? In the 1 s orbital, filling it up (each orb can only hold 2 electrons!) Orbital Diagrams Opposite spins Energy Increase

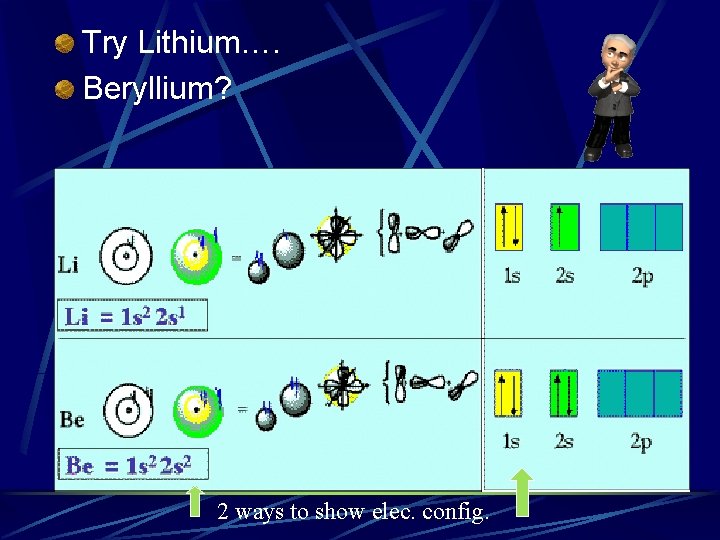
Try Lithium…. Beryllium? 2 ways to show elec. config.

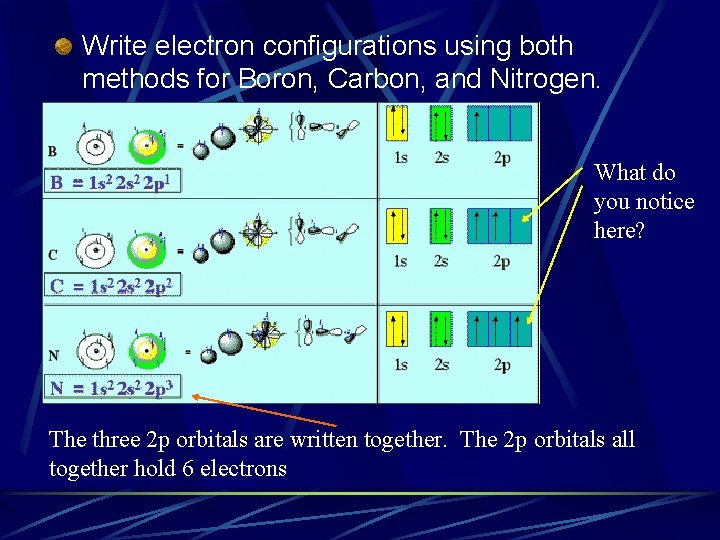
Write electron configurations using both methods for Boron, Carbon, and Nitrogen. What do you notice here? The three 2 p orbitals are written together. The 2 p orbitals all together hold 6 electrons

p Orbitals Freidrich Hund p orbitals will fill by one electron going into each p orbital (px, p. Y, pz), and then doubling up after. This is a lower energy arrangement as it minimizes electron repulsion This is called Hund’s Rule The same goes for d and f orbitals

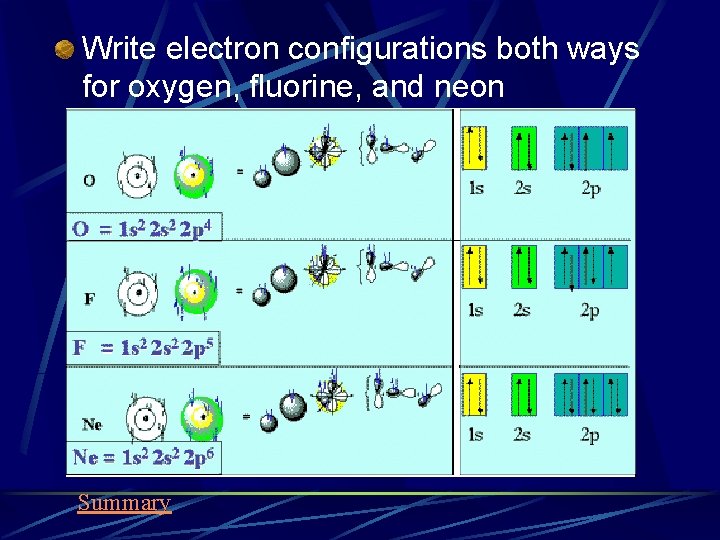
Write electron configurations both ways for oxygen, fluorine, and neon Summary

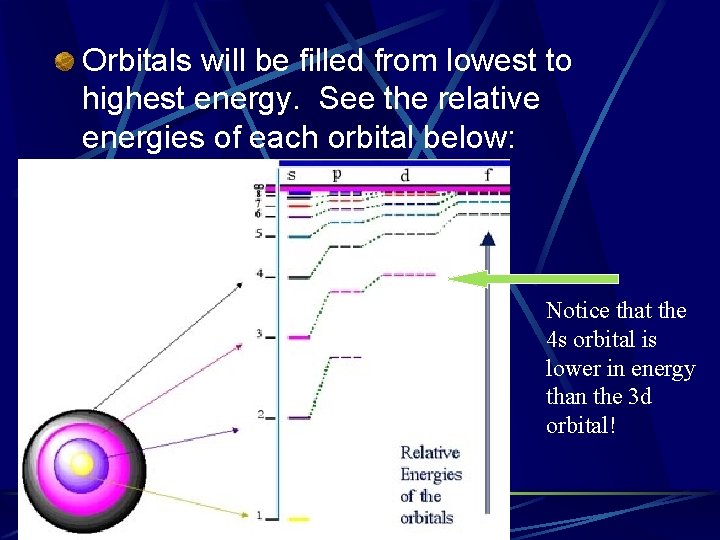
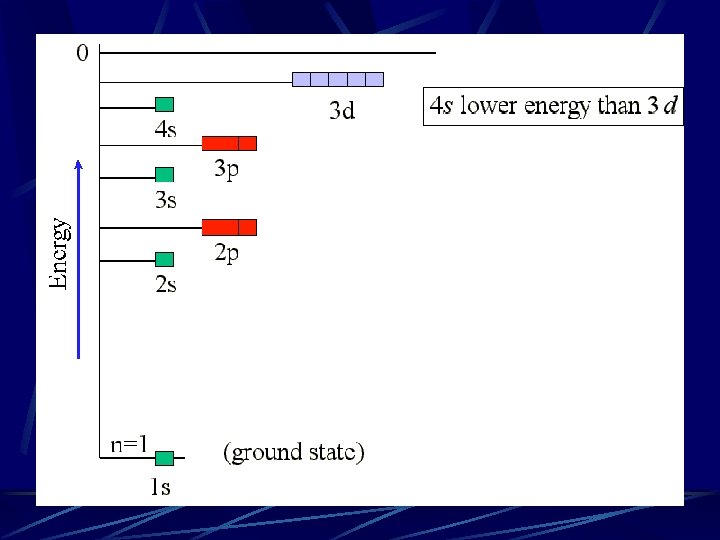
Orbitals will be filled from lowest to highest energy. See the relative energies of each orbital below: Notice that the 4 s orbital is lower in energy than the 3 d orbital!


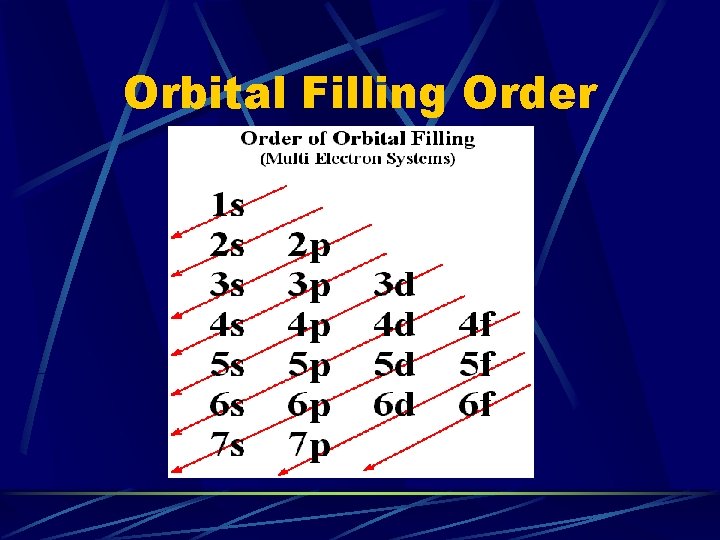
Orbital Filling Order

Practice… Give electron configurations both ways for Silicon, Calcium, and Iron…how many electrons for each? 14 for Si, 20 for Ca, 26 for Fe

E Configs and the P. Diddy What up my MD Homies!? ! Oops…I mean the P. Table

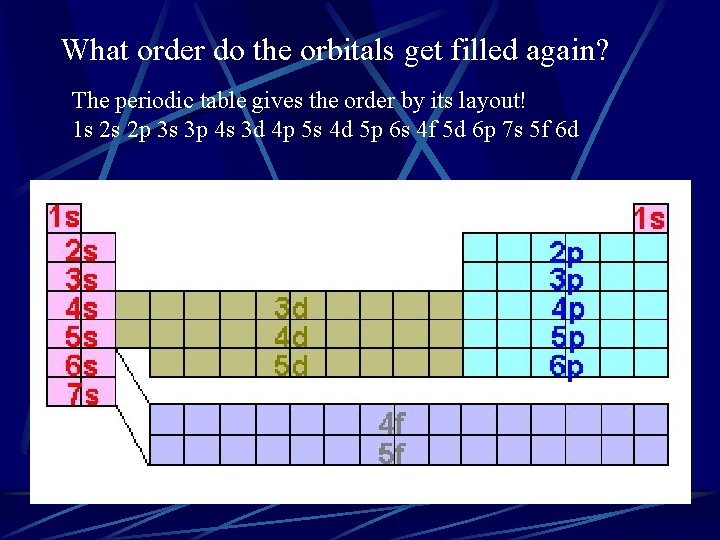
What order do the orbitals get filled again? The periodic table gives the order by its layout! 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d 5 p 6 s 4 f 5 d 6 p 7 s 5 f 6 d

Trends Cell phones and i. Pods are so hot right now!! Oh, I mean periodic table trends Give the electron configurations for hydrogen, lithium, sodium, and potassium. What is similar about each config? Correct! They all end in s 1

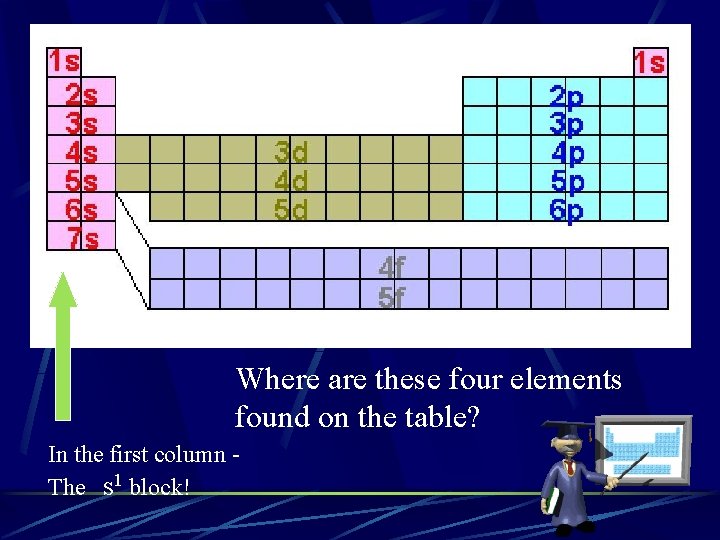
Where are these four elements found on the table? In the first column The s 1 block!

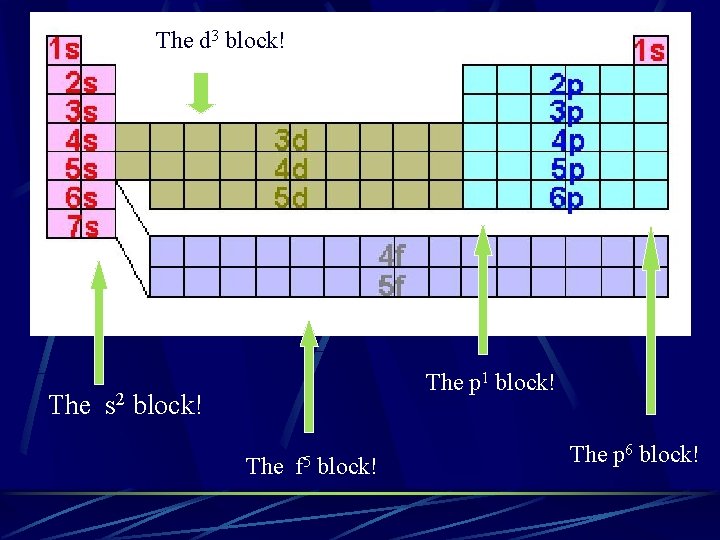
The d 3 block! The p 1 block! The s 2 block! The f 5 block! The p 6 block!

Quantum Numbers Quantum numbers are a way of keeping track of electrons Each electron in an atom has a different set of 4 quantum numbers The 1 st quantum number is called ‘n’ and tells what energy level the orbital occupies n values can be 1, 2, 3, 4, 5, 6, or 7

Keep in mind that energy and orbital size increases as the n value increases The 2 nd quantum number is ‘L’ L tells the shape of the orbital… is it an s, p, d, or f orbital? The 3 rd quantum number is ‘ml’ ml tells the orientation of the orbital… s has no orientation as it is a sphere p can be px, p. Y, or pz d and f are more complicated and you don’t have to know these

The 4 th quantum number is ‘ms’ ms gives the spin of the electron… either +1/2 or – 1/2 Electrons can have one, two, or three of the same quantum numbers, but never all four or we are describing the same electron! – Pauli Exclusion Principle Paulie “We’re gonna punch his lungs out. ” -- Paulie referring to Apollo Creed in pre-rematch interview (Rocky II)
![Core Notation Give the electron configuration for Titanium Core Notation for Ti Ar 4 Core Notation Give the electron configuration for Titanium Core Notation for Ti: [Ar] 4](https://slidetodoc.com/presentation_image_h2/144b48fcaeb6939f5dd5379cbabd7df4/image-37.jpg)
Core Notation Give the electron configuration for Titanium Core Notation for Ti: [Ar] 4 s 23 d 2 The core is the configuration that is identical to the nearest previous noble gas – then write the configuration for the remaining outer electrons

Practice… Give core notation for As, Cl, K, Cr, Ar

Core Notation for Ions If you have an anion (ion with negative charge), then the extra electrons go into the lowest energy orbital available Give core notation for P 3 P atom is [Ne] 3 s 23 p 3 The 3 - charge means three extra elecs. P 3 - is [Ne] 3 s 23 p 6 Give core notation for Cl- , O 2 -

A cation (positively charged ion) has less electrons than the neutral atom RULE: Electrons are removed from p orbitals first, then s orbitals, then d orbitals when in core notation (p before s before d) People Should Dream Give core notation for Ca 2+ First, do core notation for Ca [Ar] 4 s 2…. no p orbs outside of core, so take out the two s electrons…. therefore Ca 2+ is… [Ar]

Practice Give core notation for Fe 2+, Al 3+, Li+, Mn 2+, Fe 3+, Pb 4+ What do you notice about the electron configuration of many of the ions? They have the same configuration as noble gases! (and are happy) Ions which have identical electron configurations to noble gases are said to be isoelectronic with the noble gas

The Hulkster, like ions, in fact, is all CHARGED UP!!! ‘Mean’ Gene Okerlund…

Special Stability of Full and Half-Full d orbitals Any configuration that ends in d 4 or d 9 will undergo electron elevation, meaning an s electron moves up to a d orbital Ag [Kr] 5 s 24 d 9 is actually [Kr] 5 s 14 d 10 Ag+ [Kr] 5 s 14 d 9 is actually [Kr] 4 d 10 Cr [Ar] 4 s 23 d 4 is actually [Ar] 4 s 13 d 5

ONLY FOR d 4 and d 9 !! The elevation of an s electron to half fill or fully fill a d orbital is due to the special stability that results – it is unexpected and a phenomenon that is not well explained! Who knows?

Valence Electrons Valence electrons are the electrons that take part in chemical reactions Can lose valence electrons to become an ion (or gain valence electrons) Or, valence electrons can be used to bond with other atoms to make a compound (covalent cmpds. )

Valence electrons are all the electrons that are not in the core, and not in filled d or f orbitals How many valence electrons in each of the following? Al Ga V Xe Al 3+ S 2 -

Ions Valence electrons react in order to create a situation where an atom has filled orbitals (think about combining capacities of each chemical family)

Do your assignment or… Well… Barry Horowitz will make you… …and, believe you me…you don’t want that.