Models of the Atom In science a wrong


- Slides: 16

Models of the Atom "In science, a wrong theory can be valuable and better than no theory at all. " Sir William L. Bragg

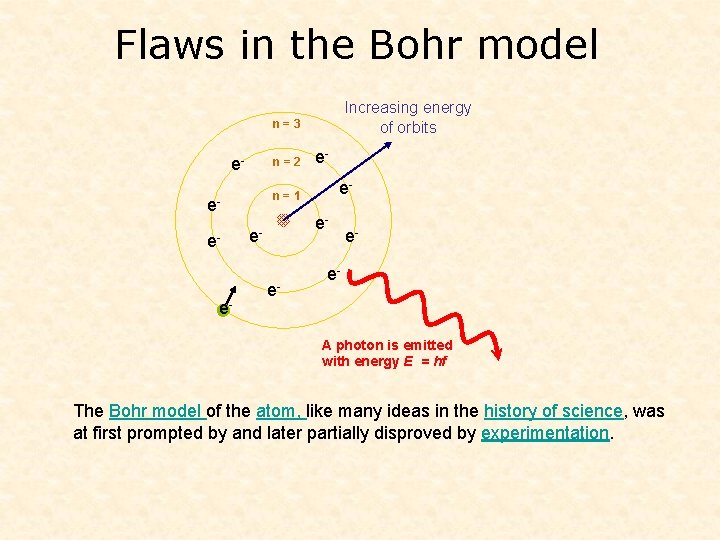
Flaws in the Bohr model Increasing energy of orbits n=3 e- n=2 e- e- n=1 ee- e- e- A photon is emitted with energy E = hf The Bohr model of the atom, like many ideas in the history of science, was at first prompted by and later partially disproved by experimentation.

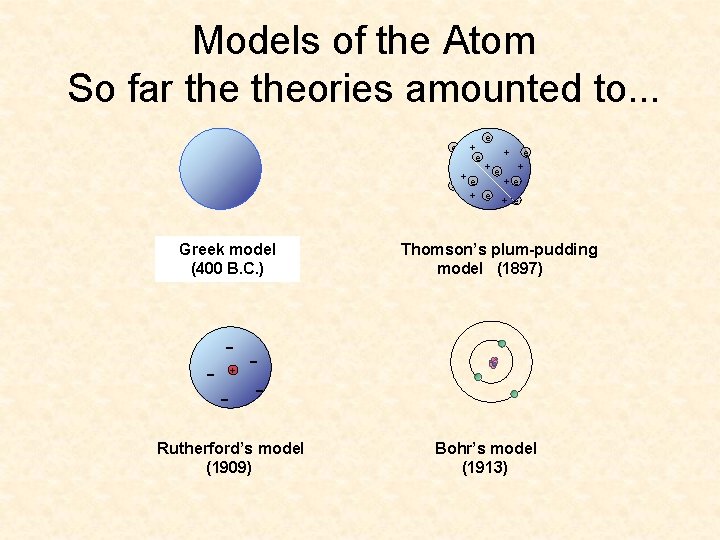
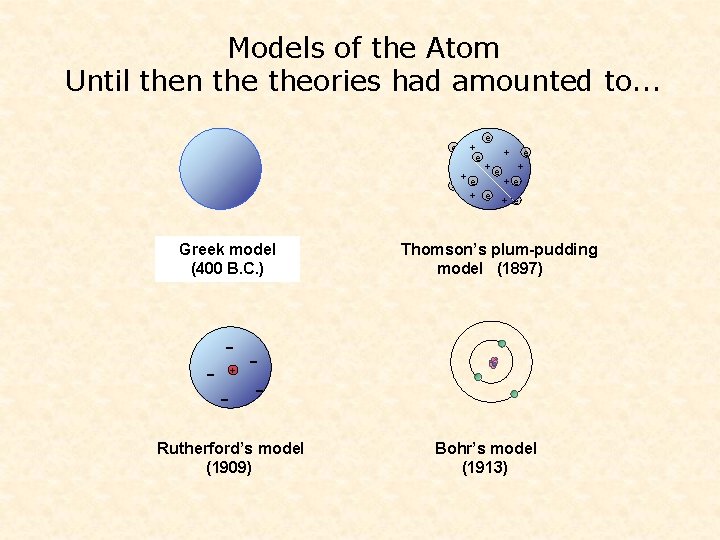
Models of the Atom So far theories amounted to. . . e + e +e +e e + e Dalton’s Greek model (400 (1803) B. C. ) Thomson’s plum-pudding model (1897) - - - + Rutherford’s model (1909) Bohr’s model (1913)

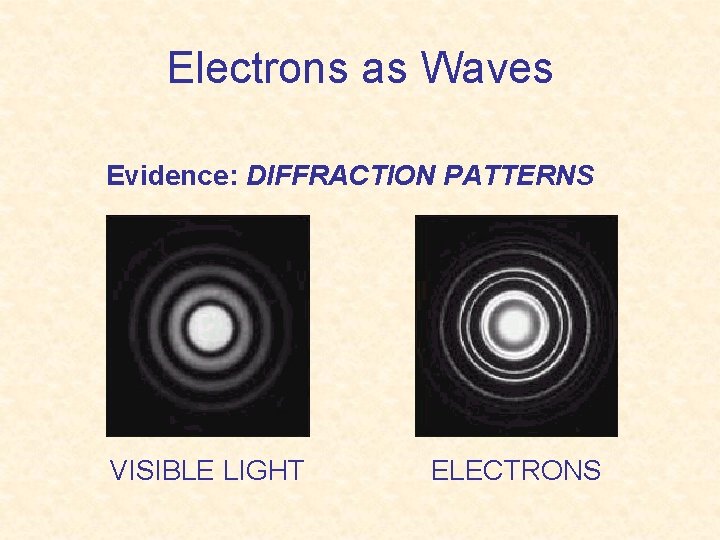
The problem was… If we pass light WAVES through a diffraction grating, we obtain a diffraction pattern VISIBLE LIGHT

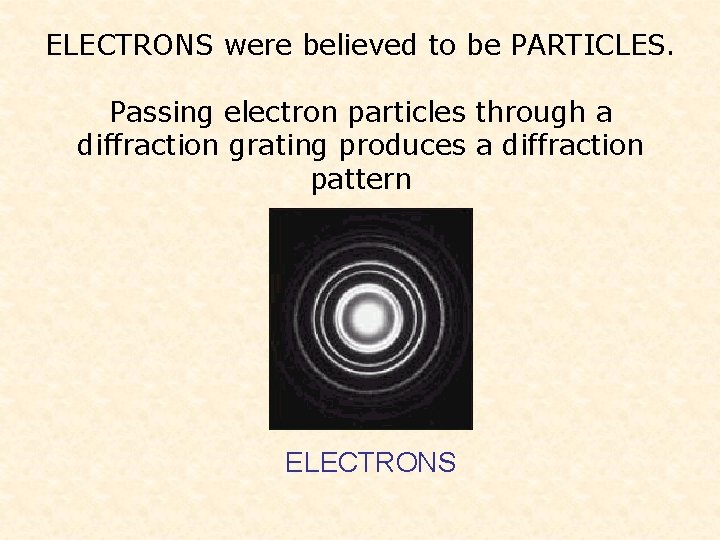
ELECTRONS were believed to be PARTICLES. Passing electron particles through a diffraction grating produces a diffraction pattern ELECTRONS

Electrons as Waves Evidence: DIFFRACTION PATTERNS VISIBLE LIGHT ELECTRONS


Models of the Atom Until then theories had amounted to. . . e + e +e +e e + e Dalton’s Greek model (400 (1803) B. C. ) Thomson’s plum-pudding model (1897) - - - + Rutherford’s model (1909) Bohr’s model (1913)

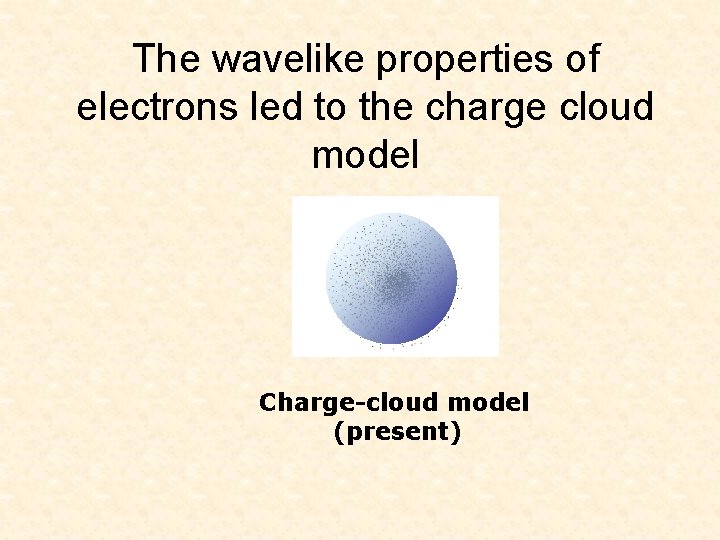
The wavelike properties of electrons led to the charge cloud model Charge-cloud model (present)

Wave – particle duality

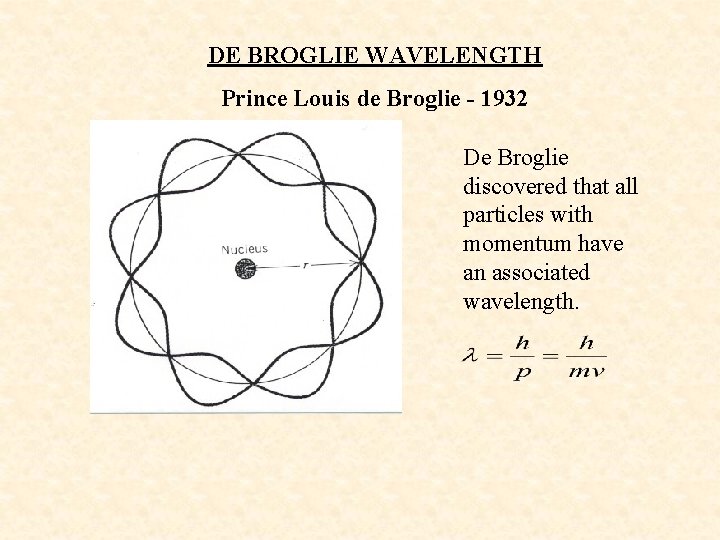
DE BROGLIE WAVELENGTH Prince Louis de Broglie - 1932 De Broglie discovered that all particles with momentum have an associated wavelength.

Erwin Schrödinger (1926) Quantum mechanics • electrons can only exist in specified energy states Electron cloud model • orbital: region around the nucleus where e- are likely to be found

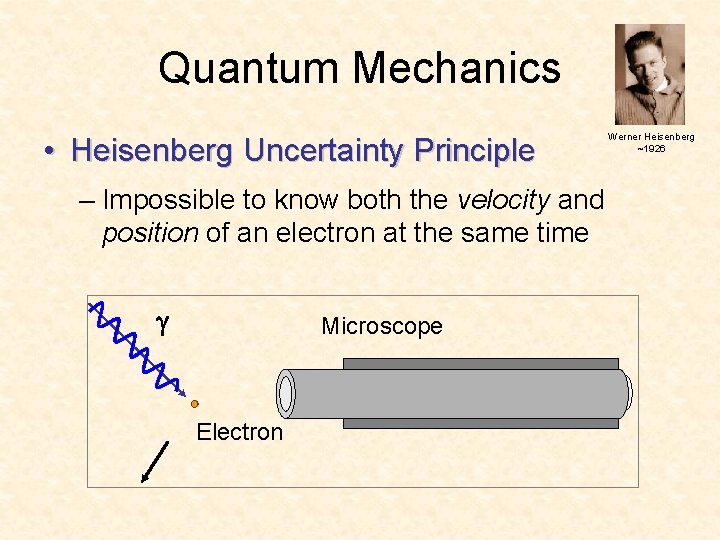
Quantum Mechanics • Heisenberg Uncertainty Principle – Impossible to know both the velocity and position of an electron at the same time g Microscope Electron Werner Heisenberg ~1926

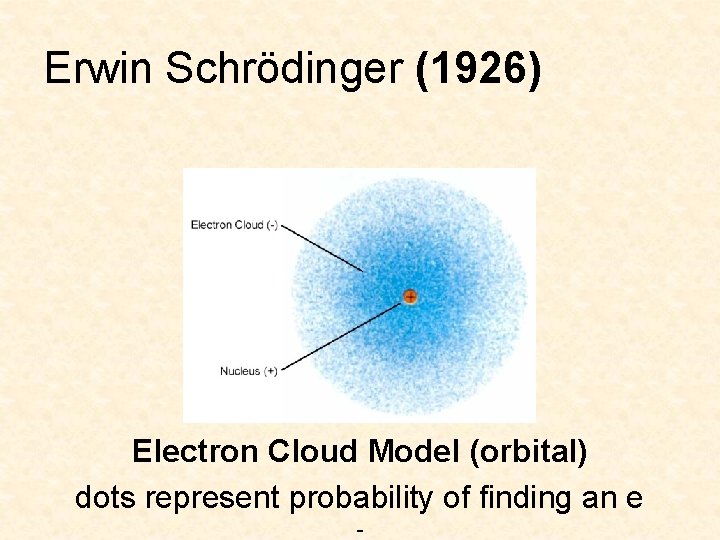
Erwin Schrödinger (1926) Electron Cloud Model (orbital) dots represent probability of finding an e -

Quantum Mechanical Model Niels Bohr & Albert Einstein Modern atomic theory describes the electronic structure of the atom as the probability of finding electrons within certain regions of space (orbitals).

Current View • The atom is mostly empty space • Two regions – Nucleus • protons and neutrons – Electron cloud • region where you might find an electron