Atomic Theory History of the Atom History of











- Slides: 33

Atomic Theory History of the Atom

History of Atom Science classroom https: //www. youtube. com/watch? v=IO 9 WS_HN myg Extra video on history of atom https: //www. youtube. com/watch? v=NSAg. Lv. KO PLQ

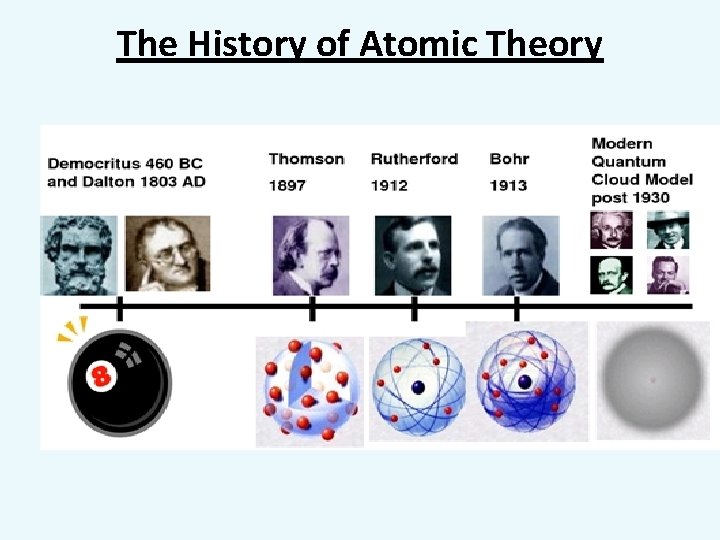
The History of Atomic Theory

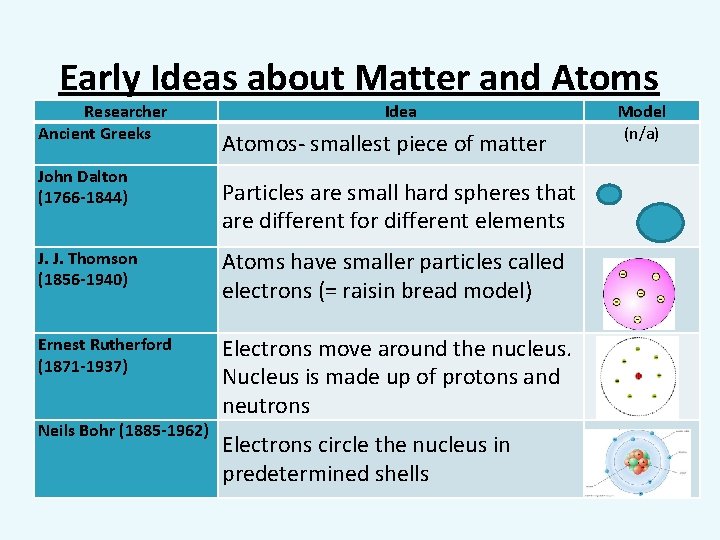
Early Ideas about Matter and Atoms Researcher Ancient Greeks John Dalton (1766 -1844) Idea Atomos- smallest piece of matter Particles are small hard spheres that are different for different elements J. J. Thomson (1856 -1940) Atoms have smaller particles called electrons (= raisin bread model) Ernest Rutherford (1871 -1937) Electrons move around the nucleus. Nucleus is made up of protons and neutrons Neils Bohr (1885 -1962) Electrons circle the nucleus in predetermined shells Model (n/a)

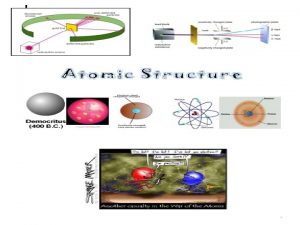
Democritus (460 BC) • Greek philosopher believed everything was made up of tiny particles in empty space. • He called them Atomos, meaning “Uncuttable”. • He did not use experiments to support his ideas, just reason and logic.

Aristotle • Philosopher Aristotle disagreed because he didn’t believe empty space could exist. • Denial of existence of atoms persisted for 2000 yr

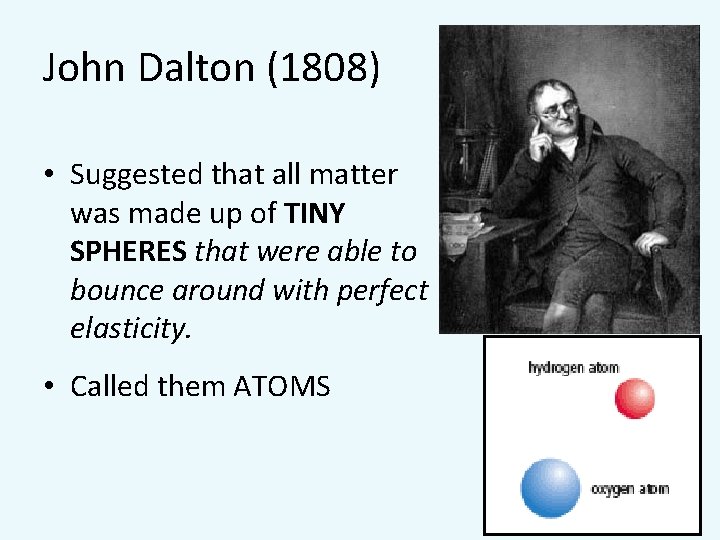
John Dalton (1808) • Suggested that all matter was made up of TINY SPHERES that were able to bounce around with perfect elasticity. • Called them ATOMS

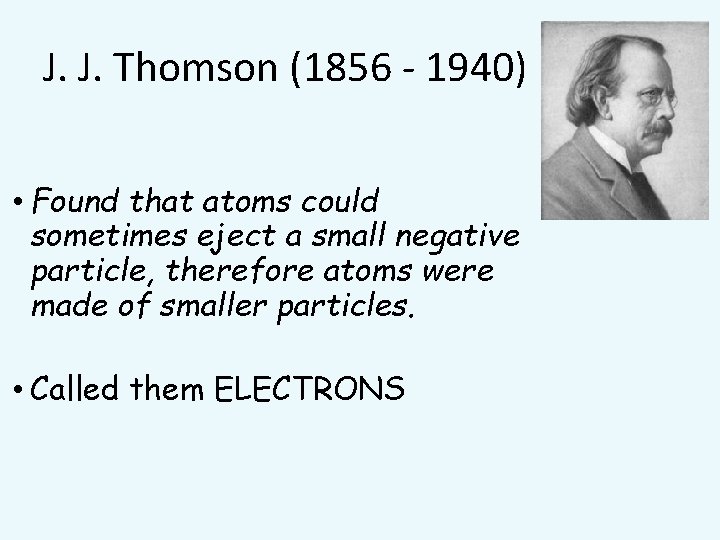
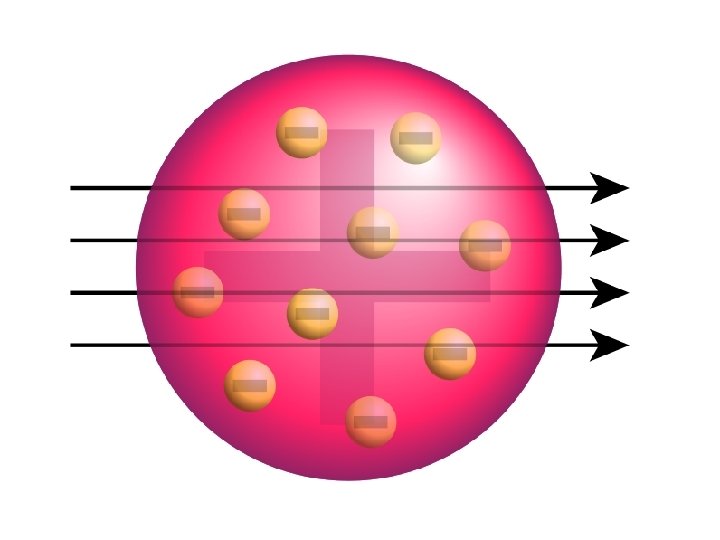
J. J. Thomson (1856 - 1940) • Found that atoms could sometimes eject a small negative particle, therefore atoms were made of smaller particles. • Called them ELECTRONS

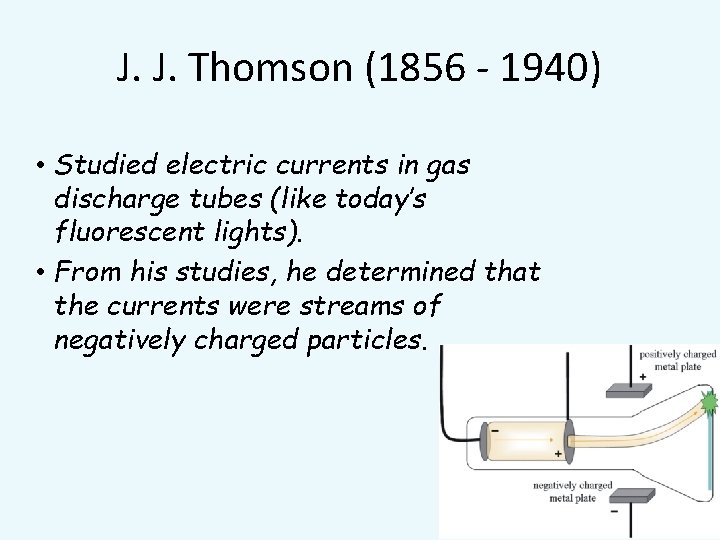
J. J. Thomson (1856 - 1940) • Studied electric currents in gas discharge tubes (like today’s fluorescent lights). • From his studies, he determined that the currents were streams of negatively charged particles.

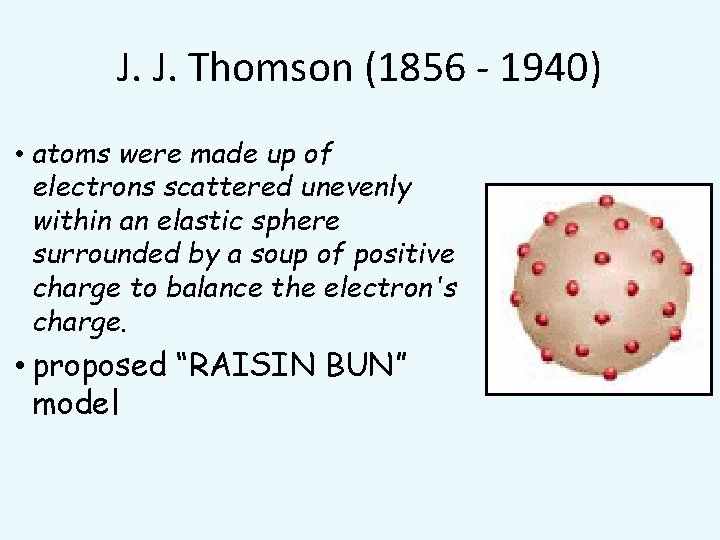
J. J. Thomson (1856 - 1940) • atoms were made up of electrons scattered unevenly within an elastic sphere surrounded by a soup of positive charge to balance the electron's charge. • proposed “RAISIN BUN” model

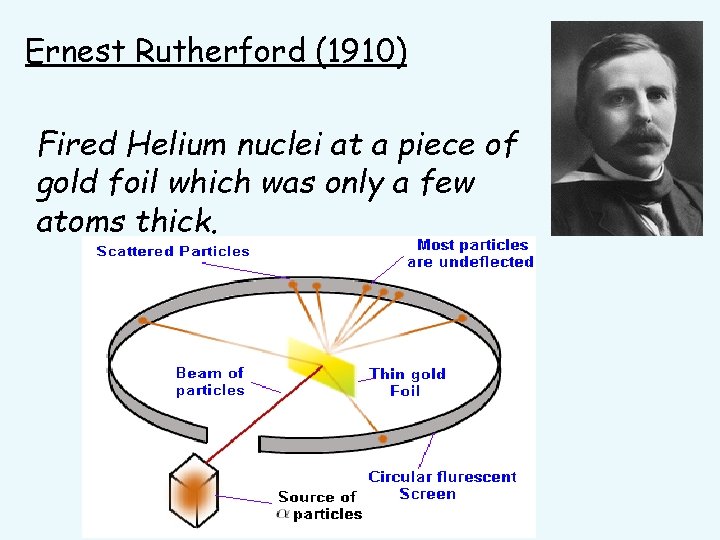
Ernest Rutherford (1910) Fired Helium nuclei at a piece of gold foil which was only a few atoms thick.




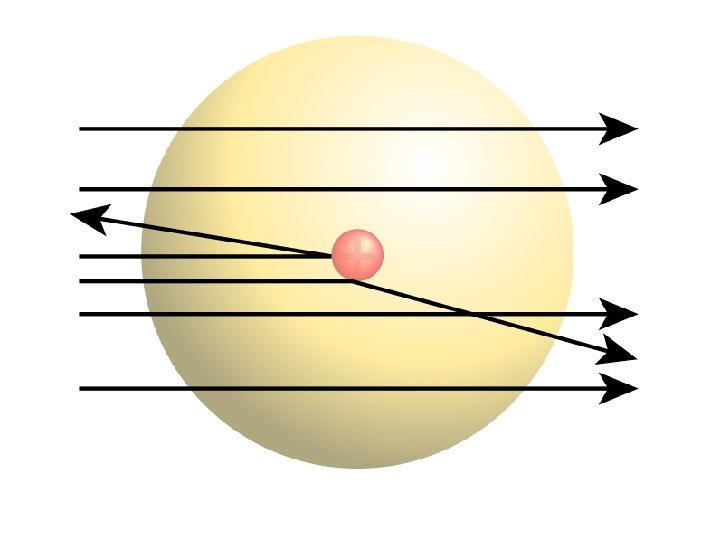
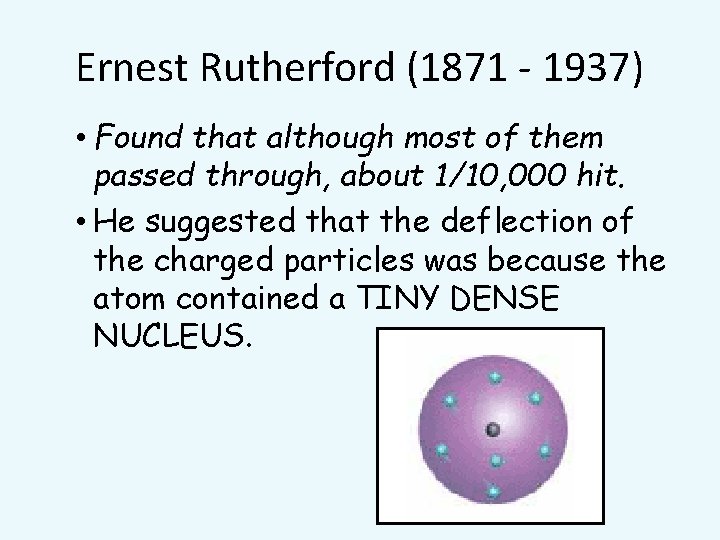
Ernest Rutherford (1871 - 1937) • Found that although most of them passed through, about 1/10, 000 hit. • He suggested that the deflection of the charged particles was because the atom contained a TINY DENSE NUCLEUS.

Rutherford’s new evidence allowed him to propose a more detailed model with a central nucleus. He suggested that the positive charge was all in a central nucleus. With this holding the electrons in place by electrical attraction.

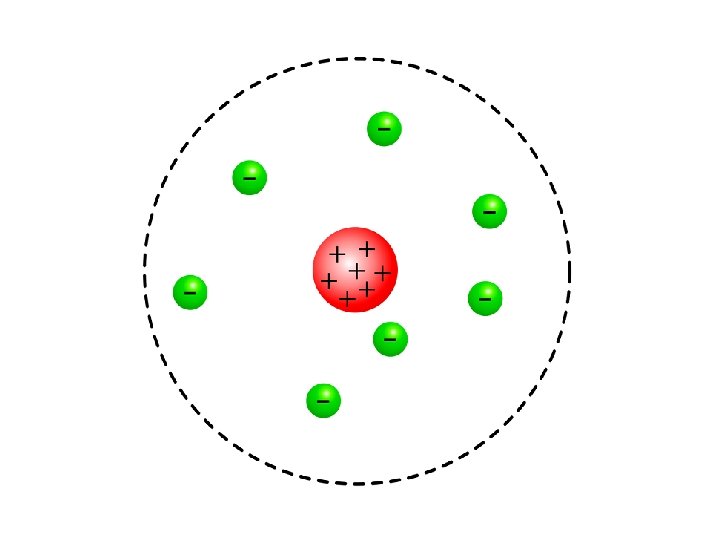
Rutherford Electrons move AROUND THE NUCLEUS. Nucleus is made up of PROTONS and NEUTRONS.

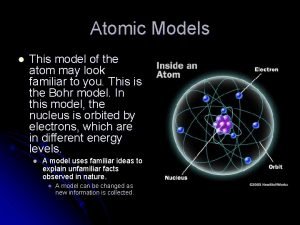
Niels Bohr (1885 - 1962) • Refined Rutherford’s idea by adding that the electrons were in ORBITS. • Proposed that electrons surround the nucleus in specific “ENERGY LEVELS” or “SHELLS. ” • Each orbit only able to contain a SET NUMBER of electrons.

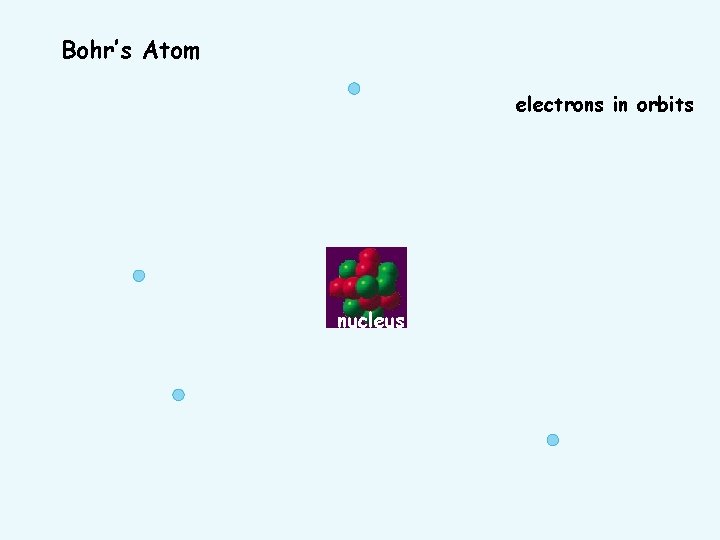
Bohr’s Atom electrons in orbits nucleus

Dalton’s Atomic Theory 1. All matter is made of small particles called atoms. 2. Atoms cannot be created, destroyed, or divided into smaller particles. 3. All atoms of the same element are identical in mass and size, but they are different in mass and size from the atoms of other elements. 4. Compounds are created when atoms of different elements link together in definite proportions.

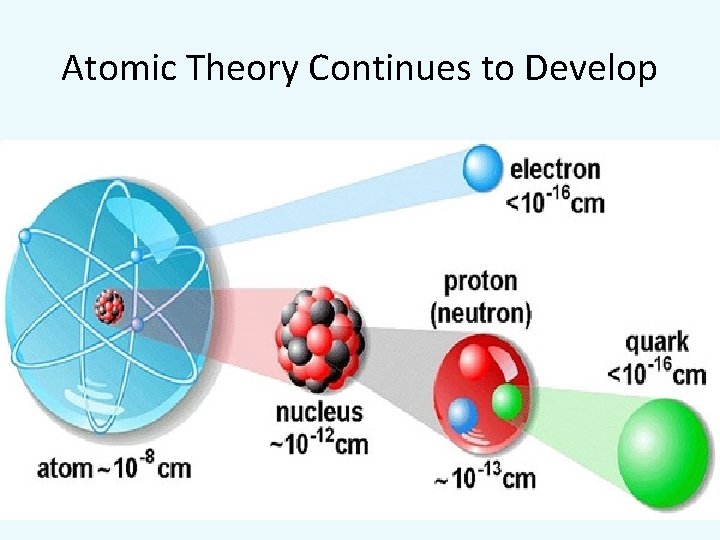
Atomic Theory Continues to Develop

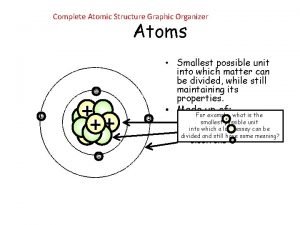
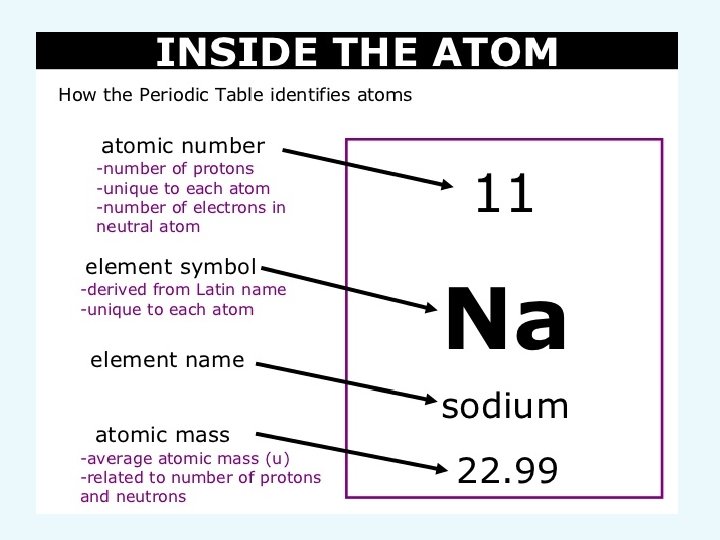
Review: What is an atom? • Atom: smallest particle of an element • Element: Pure substance that cannot be broken down into simpler substances

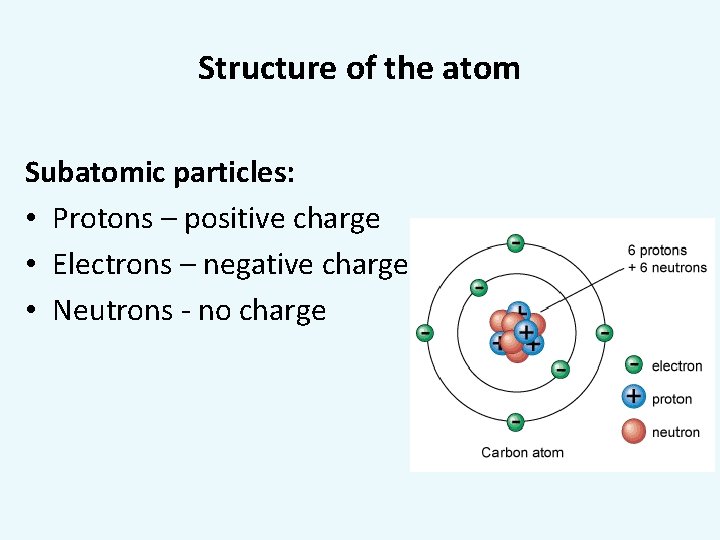
Structure of the atom Subatomic particles: • Protons – positive charge • Electrons – negative charge • Neutrons - no charge

The Atom What is an atom? https: //www. youtube. com/watch? v=o-3 I 1 JGWCk Ted Ed: How small is an atom? https: //www. youtube. com/watch? v=y. QP 4 UJh. N n 0 I

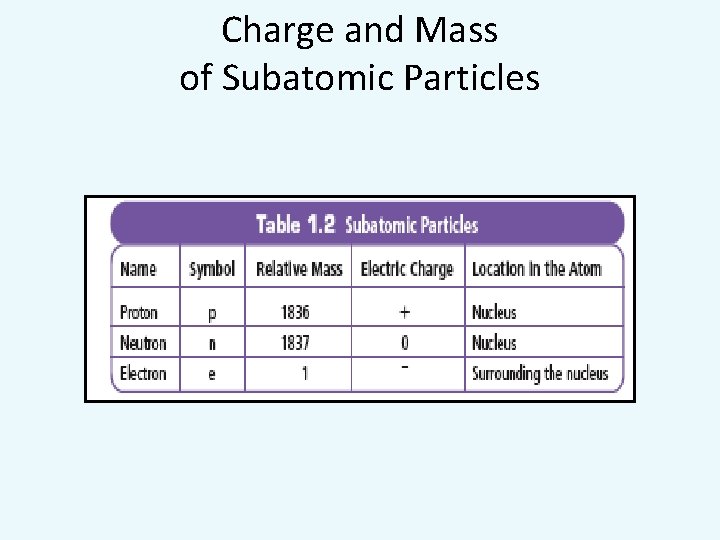
Charge and Mass of Subatomic Particles

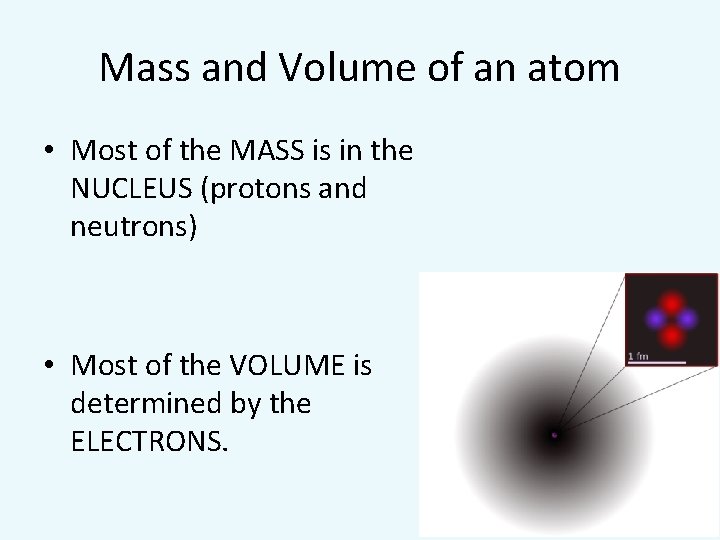
Mass and Volume of an atom • Most of the MASS is in the NUCLEUS (protons and neutrons) • Most of the VOLUME is determined by the ELECTRONS.

Check your Understanding 1. What are three subatomic particles? 2. Compare and contrast the electron and proton in terms of location and charge. 3. What accounts for an atom`s mass 4. What accounts for an atoms volume



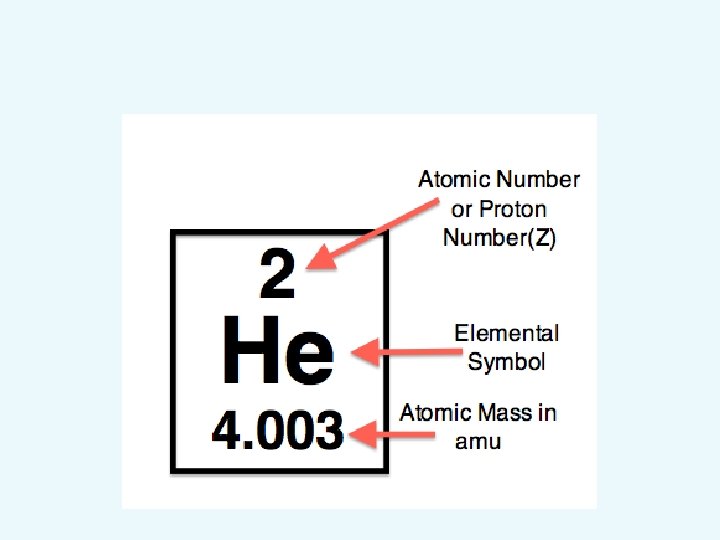
Try this: • • What is the atomic number of Oxygen ? How many protons does it have ? What is the atomic mass ? How many neutrons does it have ?

Practice Element Hydrogen Chromium Carbon Barium Iron Symbol Mass # # Protons # Neutrons

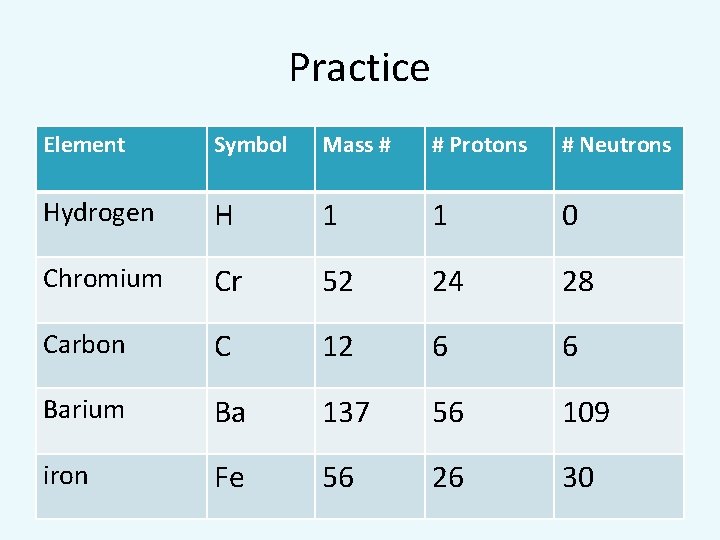
Practice Element Symbol Mass # # Protons # Neutrons Hydrogen H 1 1 0 Chromium Cr 52 24 28 Carbon C 12 6 6 Barium Ba 137 56 109 iron Fe 56 26 30

Homework • Read p 14 -15 of handout, complete atomic history chart in notes. • Answer pages 16 -18 of Work Book handout. • Read pages 204 -207 • Answer 1, 2, 5, 6, 8 -12 p 208
 History of atomic theory
History of atomic theory Timeline of atomic theory
Timeline of atomic theory Relative atomic mass of beryllium
Relative atomic mass of beryllium Radius trend periodic table
Radius trend periodic table Ion size trend
Ion size trend Isotope abundance formula
Isotope abundance formula Atomic
Atomic Atomic number vs atomic radius
Atomic number vs atomic radius Matterville worksheet
Matterville worksheet Lesson 11 atomic pudding models of the atom
Lesson 11 atomic pudding models of the atom Lesson 11 atomic pudding models of the atom answer key
Lesson 11 atomic pudding models of the atom answer key The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Democritus dalton
Democritus dalton 460+370
460+370Henry moseley atomic theory timeline
 Democritus model of atom
Democritus model of atom Niels bohr conclusion
Niels bohr conclusion Erwin schrödinger atomic theory
Erwin schrödinger atomic theory Erwin schrödinger atomic theory
Erwin schrödinger atomic theory Atomic theory timeline
Atomic theory timeline Dalton atomic theory summary
Dalton atomic theory summary Theory of atomic absorption spectroscopy
Theory of atomic absorption spectroscopy Timeline of democritus
Timeline of democritus Atomic timeline
Atomic timeline Father of atomic theory
Father of atomic theory Schrodinger atomic theory
Schrodinger atomic theory Atomic molecular theory
Atomic molecular theory Sir james chadwick atomic theory
Sir james chadwick atomic theory William crookes contribution to atomic theory
William crookes contribution to atomic theory Dalton's atomic theory was accepted because
Dalton's atomic theory was accepted because Erwin schrödinger atomic theory
Erwin schrödinger atomic theory Complete atom
Complete atom Summarize dalton's atomic theory
Summarize dalton's atomic theory Dalton thomson rutherford bohr timeline
Dalton thomson rutherford bohr timeline