THE ATOM What is the atom Atom Smallest







- Slides: 13

THE ATOM

What is the atom? • Atom • Smallest particle of an element that retains its identity in a chemical reaction • Democritus, 460 B. C. , a philosopher • First to suggest atoms exist • Main thought • Atoms were indivisible and indestructible • Lacked scientific support • Democritus helped pave the way to what we know about the atom now

John Dalton • English chemist and schoolteacher • Took Democritus’ ideas and used experimentation to help back them up • Came up with Dalton’s Atomic Theory • All elements are composed of tiny indivisible particles called atoms • Atoms of the same element are identical. Atoms of different elements are different. • Atoms of different elements can physically mix together or can chemically combine in whole numbers to form compounds. • Chemical reactions occur when atoms are separated, joined, or rearranged.

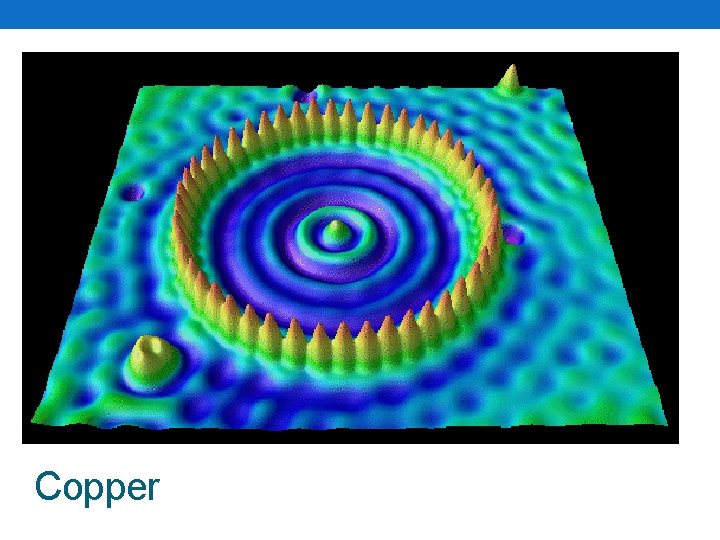
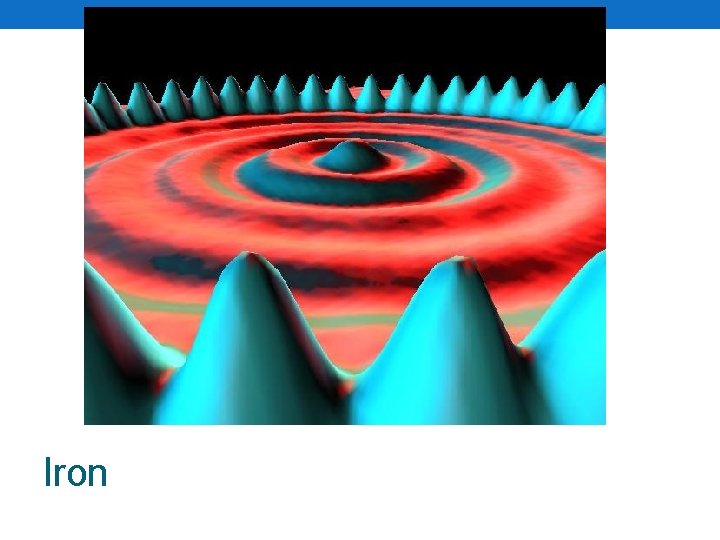
How big is an atom? • Very, very small • Lets take a copper penny • 1 copper penny • Has 24, 000, 000, 000 (sextillion) atoms in it! • Earth’s population • 7, 000, 000 people • So what? • Take 100, 000 copper atoms side by side • How big would that be? • 1 cm long! • How do we know they exist? • Scanning tunneling microscopes

Copper

Iron

Carbon Monoxide Man

Subatomic Particles • Atoms can be broken down • Called subatomic particles • Electron • Protons • Neutrons

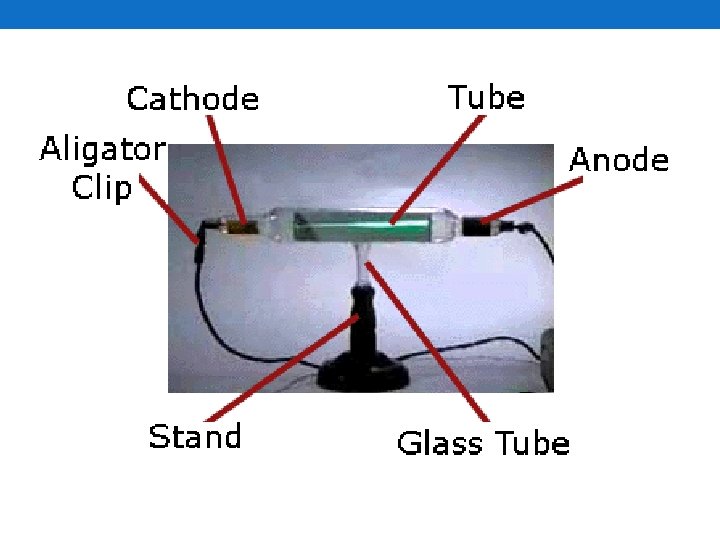
Electrons • Discovered by J. J. Thomson in 1897 • Negatively-charged particles • His experiment • Used the cathode ray tube • Noticed that positively-charged magnets attracted the cathode ray • Negatively-charged magnets deflected it • Called the particles that made up the cathode ray corpuscles • Later called electrons • Have 1 negative charge per electron • Mass • 1/1840 the mass of a proton


Protons and Neutrons • Protons • Discovered in 1886 by Eugen Goldstein • Noticed that some particles went the other way in the cathode ray tube • Called them protons • Mass • 1840 times the mass of an electron • Neutrons • Discovered in 1932 by James Chadwick • Have no charge • Neutral

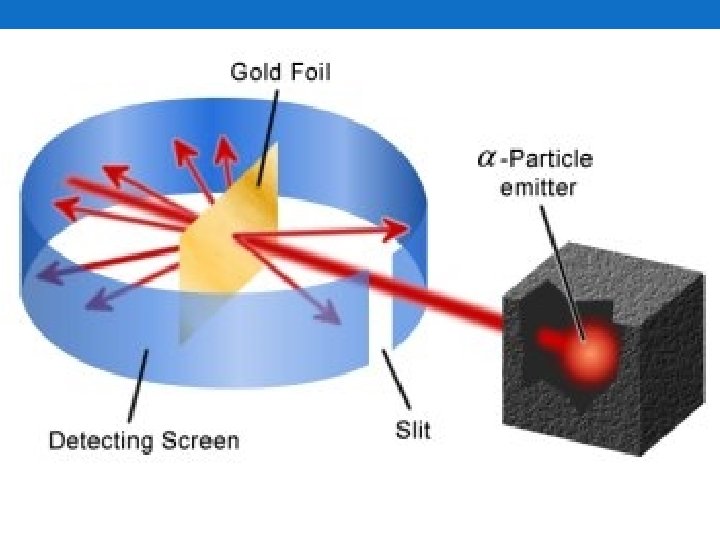
The Atomic Nucleus • We know that all these particles exist • How are they joined together? • Ernest Rutherford (1911) answered that • Gold Foil Experiment • Shot Helium atoms that lost both electrons at a piece of gold foil • Expected all the particles to pass through the gold foil • What happened? • Most passed through • Some were deflected at large angles • Some came straight back • What Rutherford concluded from this • Atoms are mostly empty space • Most of the mass is concentrated in the middle (positively charged) • He called it the nucleus • Atomic Model we accept today • Nucleus is composed of protons and neutrons • Electrons occupy a space around the nucleus
