ATOMS Atomic Theory DEFINING THE ATOM An atom









- Slides: 26

ATOMS & Atomic Theory

DEFINING THE ATOM An atom is the smallest particle of an element that retains its identity in a reaction. The basic building blocks of matter that make-up everyday objects.

ATOMIC THEORY Democritus was an early Greek Scholar. He was the first to suggest the existence of atoms Democritus believed that atoms were indivisible and indestructible. He never developed a theory because he did not have experimental support nor did he explain chemical behavior. It took 2000 years after Democritus for the real nature of atoms and events at the atomic level to be established

DALTON’S ATOMIC THEORY Using experimental methods, Dalton transformed Democritus’s ideas on atoms into a scientific theory 1. All matter is composed of tiny indivisible particles called atoms 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms from different elements can physically mix together or can chemically combine in simple wholenumber ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined, or rearranged. Atoms of one element, however, are never changed into atoms of another element as a result of a chemical reaction.

DALTON’S THEORY REVISED Most of Dalton’s theory is still accepted today EXCEPT that atoms are known to be divisible. Atoms can be broken down into 3 subatomic particles: electrons, protons and neutrons.

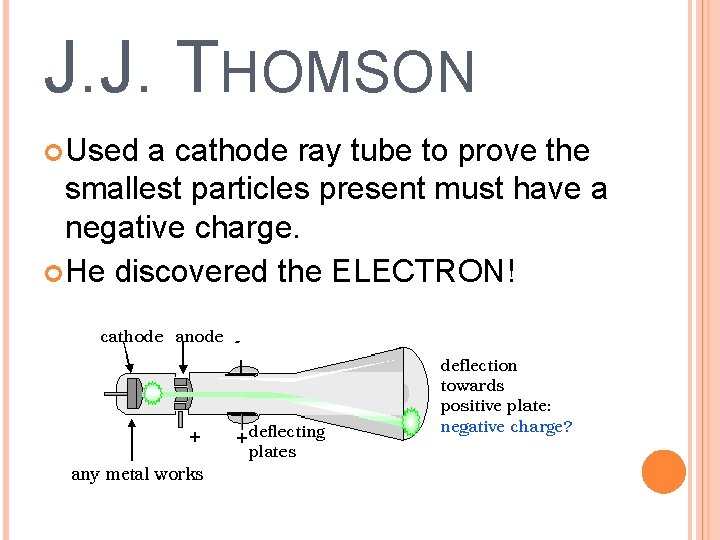
J. J. THOMSON Used a cathode ray tube to prove the smallest particles present must have a negative charge. He discovered the ELECTRON!

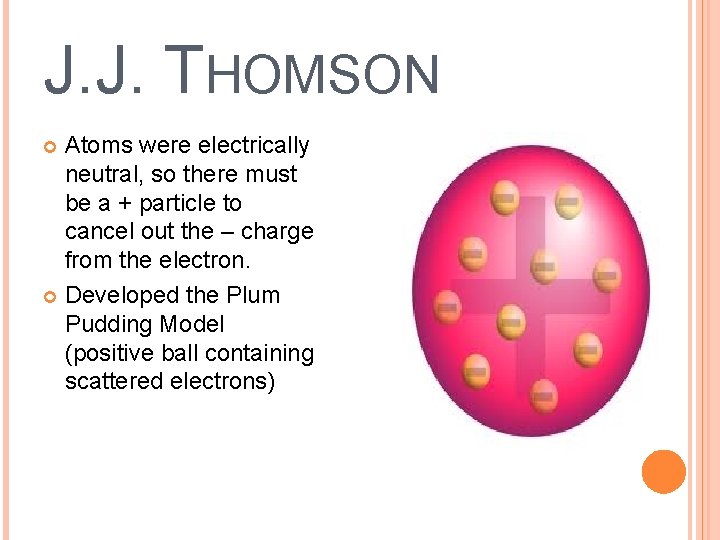
J. J. THOMSON Atoms were electrically neutral, so there must be a + particle to cancel out the – charge from the electron. Developed the Plum Pudding Model (positive ball containing scattered electrons)

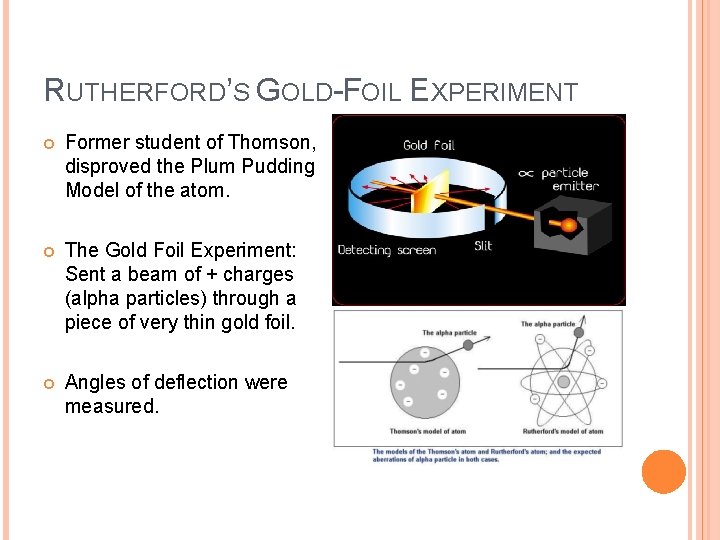
RUTHERFORD’S GOLD-FOIL EXPERIMENT Former student of Thomson, disproved the Plum Pudding Model of the atom. The Gold Foil Experiment: Sent a beam of + charges (alpha particles) through a piece of very thin gold foil. Angles of deflection were measured.

RUTHERFORD Results: Most of the alpha particles passed straight through, most of the foil must be regions of “empty” space – not a + sphere like Thomson believed. + charges and the atoms mass must be found in the center discovered the nucleus

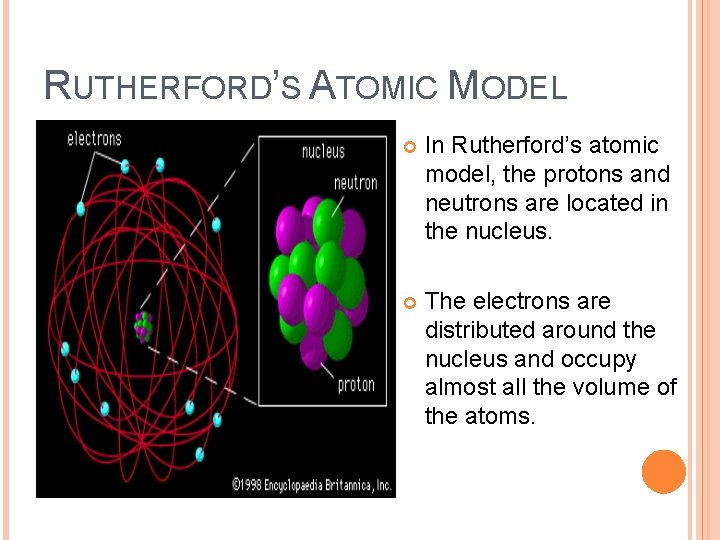
RUTHERFORD’S ATOMIC MODEL In Rutherford’s atomic model, the protons and neutrons are located in the nucleus. The electrons are distributed around the nucleus and occupy almost all the volume of the atoms.

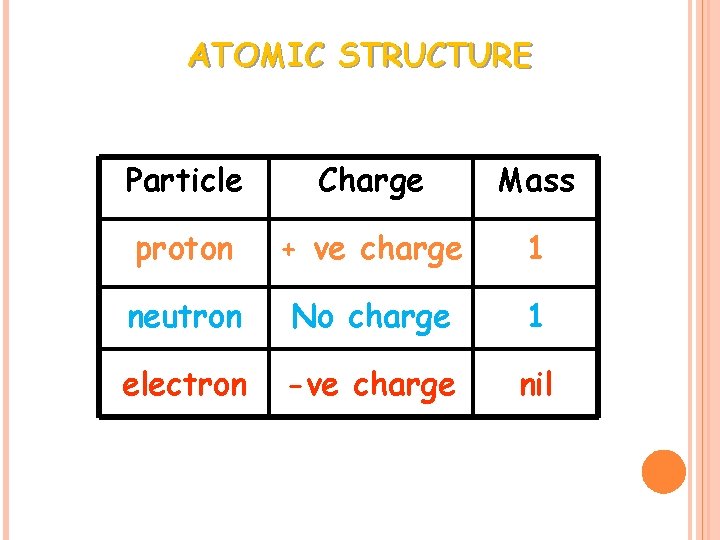
ATOMIC STRUCTURE Particle Charge Mass proton + ve charge 1 neutron No charge 1 electron -ve charge nil

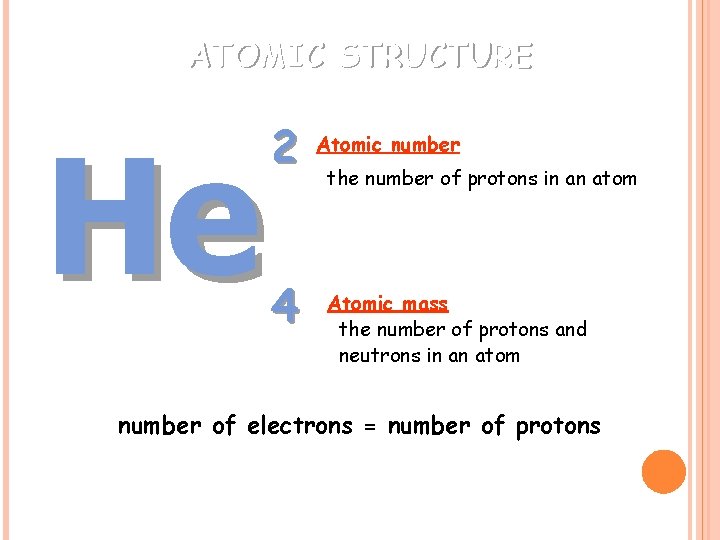
ATOMIC STRUCTURE He 2 4 Atomic number the number of protons in an atom Atomic mass the number of protons and neutrons in an atom number of electrons = number of protons

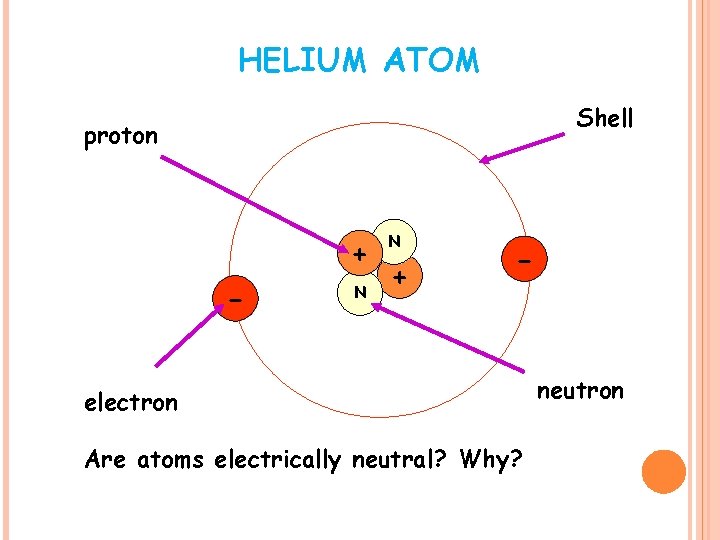
HELIUM ATOM Shell proton + - N N + - electron Are atoms electrically neutral? Why? neutron

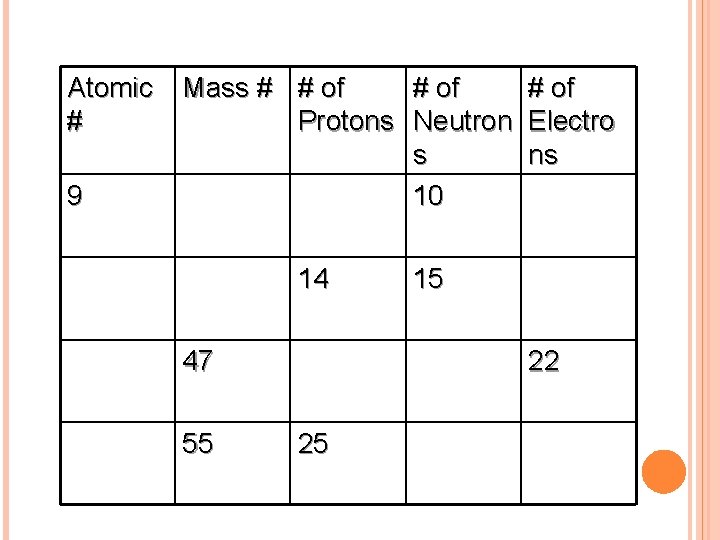
COMPLETE THE FOLLOWING TABLE IN YOUR NOTES Atomic Mass # # of # Protons Neutron Electro s ns 9 10 14 47 55 15 22 25

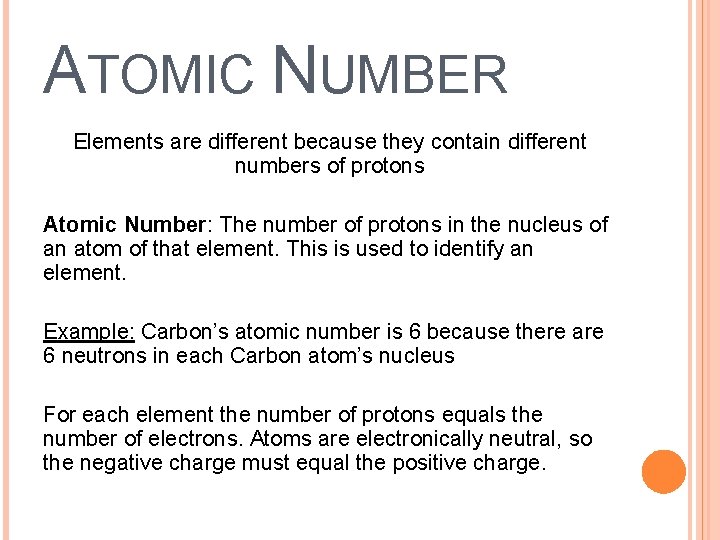
ATOMIC NUMBER Elements are different because they contain different numbers of protons Atomic Number: The number of protons in the nucleus of an atom of that element. This is used to identify an element. Example: Carbon’s atomic number is 6 because there are 6 neutrons in each Carbon atom’s nucleus For each element the number of protons equals the number of electrons. Atoms are electronically neutral, so the negative charge must equal the positive charge.

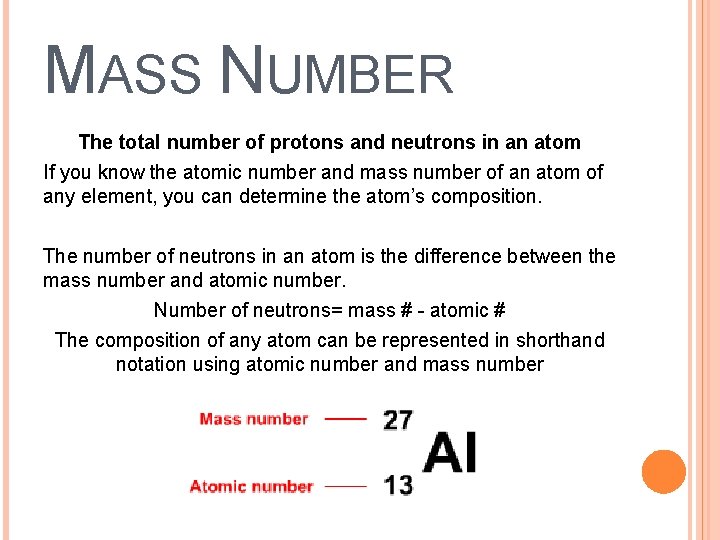
MASS NUMBER The total number of protons and neutrons in an atom If you know the atomic number and mass number of an atom of any element, you can determine the atom’s composition. The number of neutrons in an atom is the difference between the mass number and atomic number. Number of neutrons= mass # - atomic # The composition of any atom can be represented in shorthand notation using atomic number and mass number

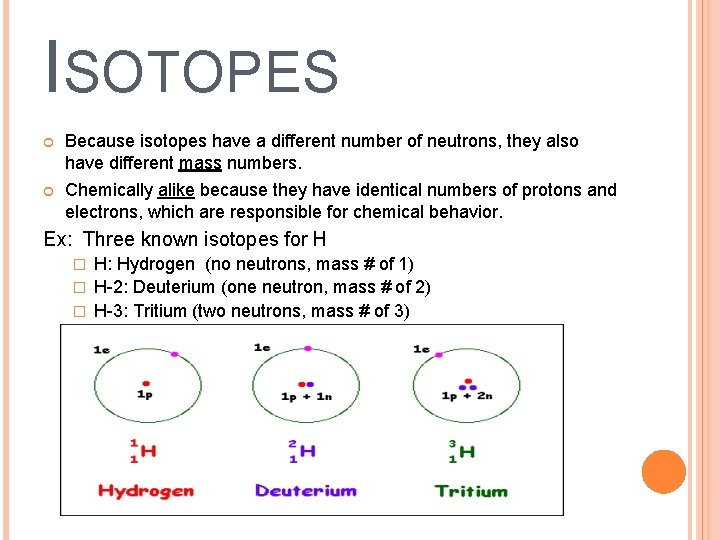
ISOTOPES Atoms that have the same number of protons but different numbers of neutrons

ISOTOPES Because isotopes have a different number of neutrons, they also have different mass numbers. Chemically alike because they have identical numbers of protons and electrons, which are responsible for chemical behavior. Ex: Three known isotopes for H H: Hydrogen (no neutrons, mass # of 1) � H-2: Deuterium (one neutron, mass # of 2) � H-3: Tritium (two neutrons, mass # of 3) �

CALCULATING AVERAGE ATOMIC MASS OF ISOTOPES In nature, isotopes occur in various percentages. The higher the percent the more abundant. In order to figure out the average mass of each element the percent abundance and mass of each isotope need to be considered We can calculate average atomic mass in much the same way as we calculate your grade in this class…

CALCULATING AVERAGE ATOMIC MASS 1. Divide the percent abundances by 100. (natural occurrence) 2. Multiply each isotope mass by its natural occurrence. (maintain sigfigs)* 3. Add up all the masses (maintain place values) 4. Include a unit (amu)

WHAT ARE THE DIFFERENT CATEGORIES THAT YOU ARE GRADED ON IN THIS CLASS? Classwork: 79 pts Practice: 12 pts Final: 14 pts What would your semester grade be if you received an 81% for classwork, 52% for practice, and 73% on your final? � 0. 81 x 79 = 64 � 0. 52 x 12 = 6. 2 � 0. 73 x 14 = 10 � Add all answers together to get % semester grade � 63. 2 + 3. 5 + 10. 1 = 80. 2 80 % (a B)

NOW LETS TRY WITH AN ELEMENT! Copper has two isotopes: copper-63 and copper-65. The relative abundances of these isotopes are 69. 2% and 30. 8% respectively. Calculate the average atomic mass of copper. 0. 692 x 63 = 43. 6 0. 308 x 65 = 20. 0 43. 6 + 20. 0 = 63. 6

ONE MORE EXAMPLE… Uranium has three naturally occurring isotopes with the following percent abundances: U-234 (0. 0058%), U-235 (0. 71%), and U-238 (99. 23%). � What do you expect the average atomic mass to be and why? � What is the average atomic mass? 237. 9

SUMMARY 1. The Atomic Number of an atom = number of protons in the nucleus. 2. The Atomic Mass of an atom = number of Protons + Neutrons in the nucleus. 3. The number of Protons = Number of Electrons. 4. Electrons orbit the nucleus in shells. 5. Each shell can only carry a set number of electrons.

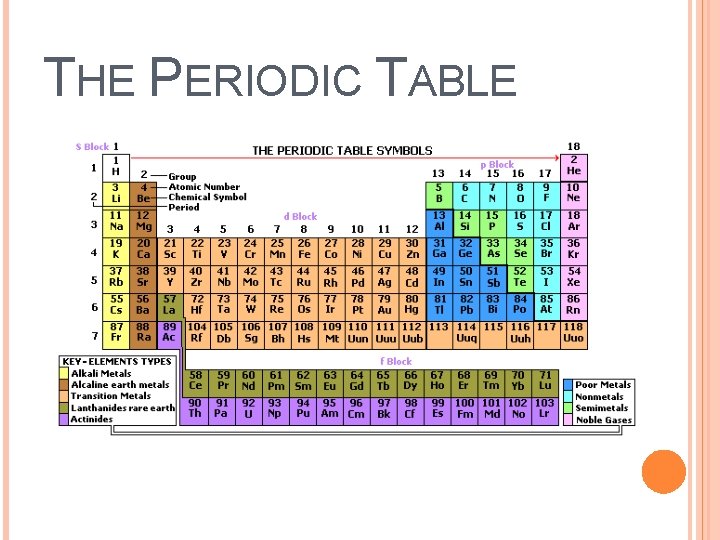
THE PERIODIC TABLE A periodic table allows you to easily compare the properties of one element (or a group of elements) to another element (or group of elements) Period- Each horizontal row of the periodic table. The properties of the elements vary as you move across it from element to element. Group (or family)- Each vertical column of the periodic table. Elements within a group have similar chemical and physical properties.

THE PERIODIC TABLE