HISTORY OF THE ATOMIC THEORY HISTORY OF ATOMIC













- Slides: 17

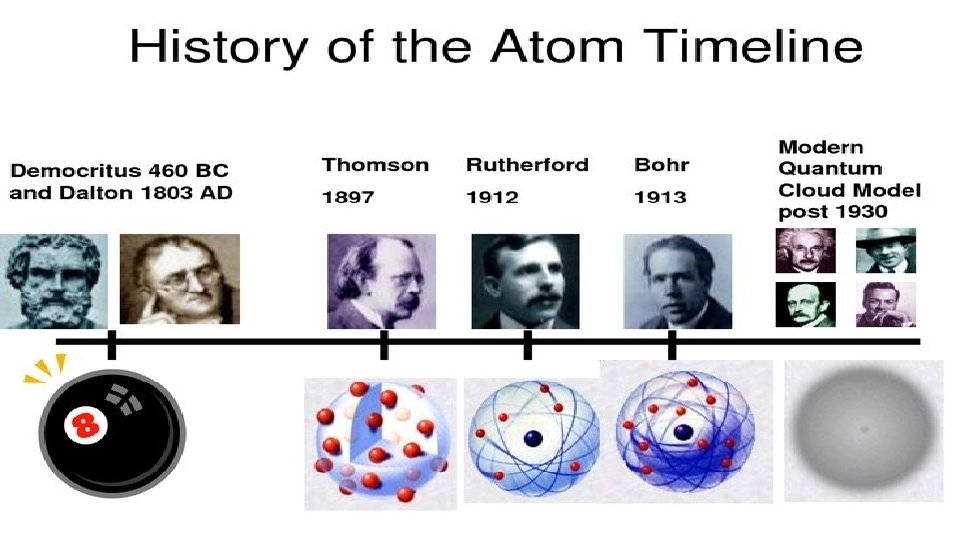
HISTORY OF THE ATOMIC THEORY

HISTORY OF ATOMIC THEORY • HTTPS: //WWW. YOUTUBE. COM/WATCH? V= IO 9 WS_HNMYG


ATOMIC STRUCTURE PG. 23

THE ATOM • SMALLEST FORM OF AN ELEMENT THAT STILL RETAINS THAT ELEMENTS PROPERTIES • BASIC BUILDING BLOCK OF MATTER • MADE OF: PROTONS, NEUTRONS AND ELECTRONS • COMPOSED OF: NUCLEUS AND ELECTRON CLOUD

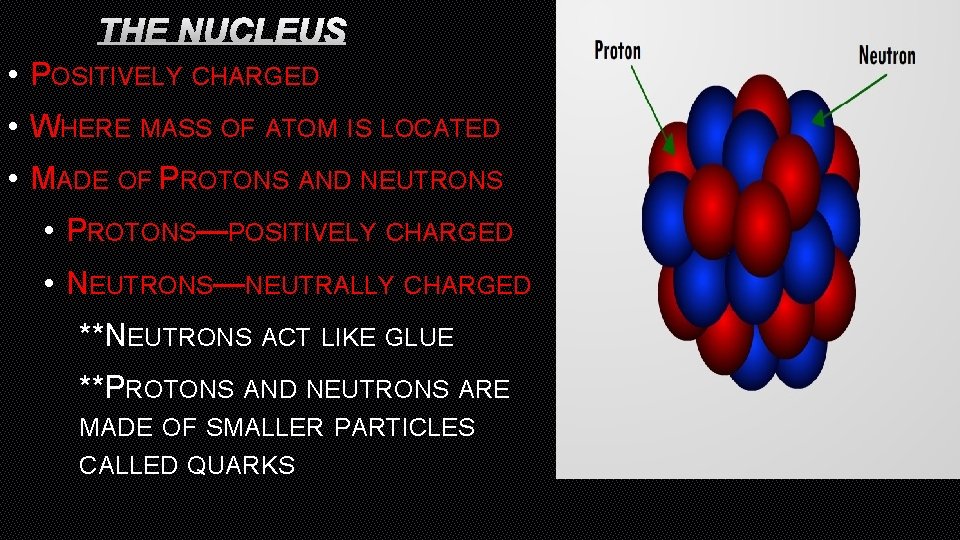
THE NUCLEUS • POSITIVELY CHARGED • WHERE MASS OF ATOM IS LOCATED • MADE OF PROTONS AND NEUTRONS • PROTONS—POSITIVELY CHARGED • NEUTRONS—NEUTRALLY CHARGED **NEUTRONS ACT LIKE GLUE **PROTONS AND NEUTRONS ARE MADE OF SMALLER PARTICLES CALLED QUARKS

THE ELECTRONS • NEGATIVELY CHARGED • OCCUPY THE “ELECTRON CLOUD” SURROUNDING THE NUCLEUS • ELECTRONS ARE NOT MOVING IN PERFECT ORBITS AROUND NUCLEUS AND ARE UNPREDICTABLE SO CLOUD GIVES US APPROXIMATE LOCATION OF WHERE ELECTRONS ARE


SUBATOMIC PARTICLES


PROPERTIES OF ATOMS INSIDE THE SQUARES…

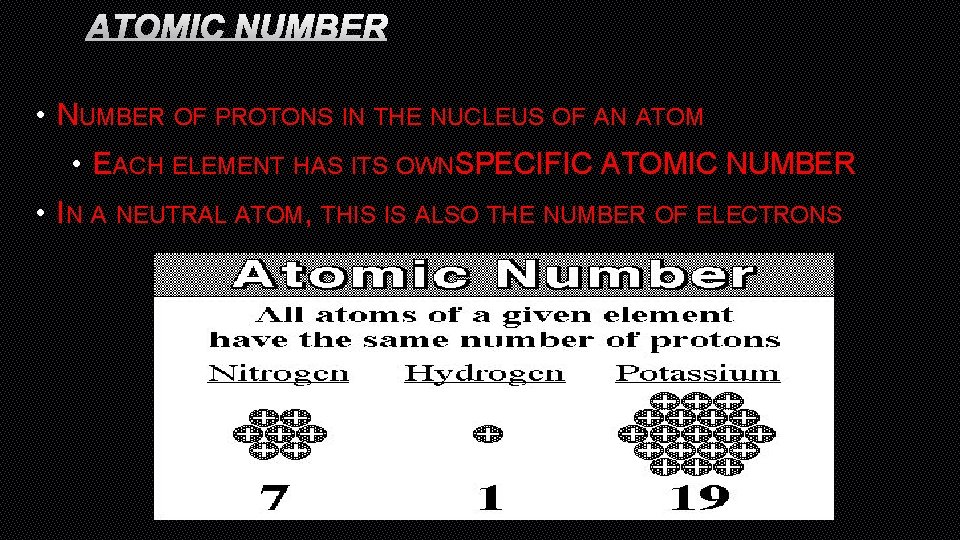
ATOMIC NUMBER • NUMBER OF PROTONS IN THE NUCLEUS OF AN ATOM • EACH ELEMENT HAS ITS OWNSPECIFIC ATOMIC NUMBER • IN A NEUTRAL ATOM, THIS IS ALSO THE NUMBER OF ELECTRONS

MASS NUMBER • SUM OF THE PROTONS AND NEUTRONS IN THE NUCLEUS OF A SPECIFIC ISOTOPE OF AN ATOM • MASS NUMBER OF SPECIFIC ISOTOPE= #NEUTRONS + #PROTONS

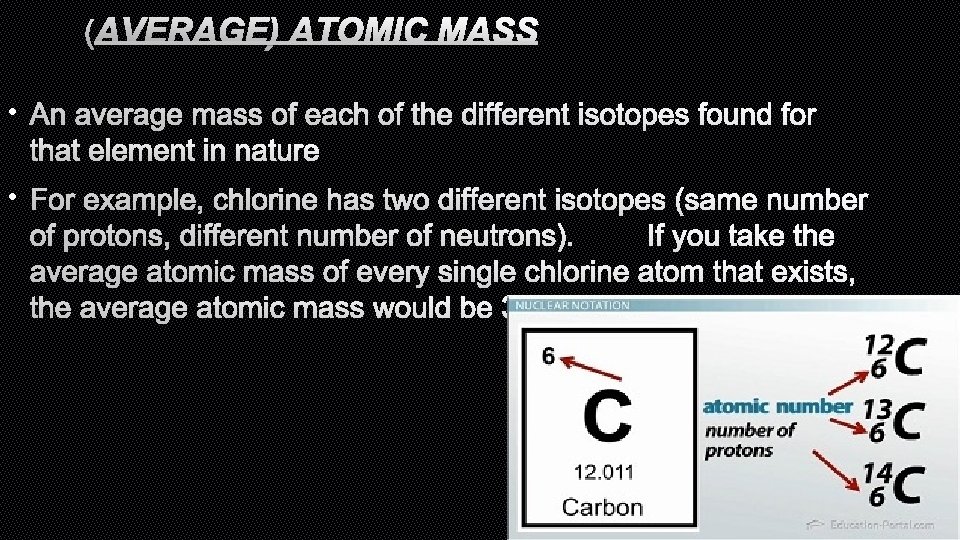
(AVERAGE) ATOMIC MASS • AN AVERAGE MASS OF EACH OF THE DIFFERENT ISOTOPES FOUND FOR THAT ELEMENT IN NATURE • FOR EXAMPLE, CHLORINE HAS TWO DIFFERENT ISOTOPES (SAME NUMBER OF PROTONS, DIFFERENT NUMBER OF NEUTRONS). IF YOU TAKE THE AVERAGE ATOMIC MASS OF EVERY SINGLE CHLORINE ATOM THAT EXISTS, THE AVERAGE ATOMIC MASS WOULD BE 35. 453 AMU



ATOMIC BASICS VIDEOS • HTTPS: //WWW. YOUTUBE. COM/WATCH? V= EMDRB 2 LQL 7 E • HTTPS: //WWW. YOUTUBE. COM/WATCH? V=S RPEJONKTKE
 1930 modern atom teorisi
1930 modern atom teorisi Plato atomic theory timeline
Plato atomic theory timeline Relative formula mass of hcl
Relative formula mass of hcl Trends periodic table
Trends periodic table Atomic trends
Atomic trends How to calculate abundance in isotopes
How to calculate abundance in isotopes Differentiate between atomic number and mass number
Differentiate between atomic number and mass number Atomic number vs atomic radius
Atomic number vs atomic radius Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Glasgow thang điểm
Glasgow thang điểm Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới