Atomic Theory Atomic Theories Timeline Use a graphic


















- Slides: 45

Atomic Theory

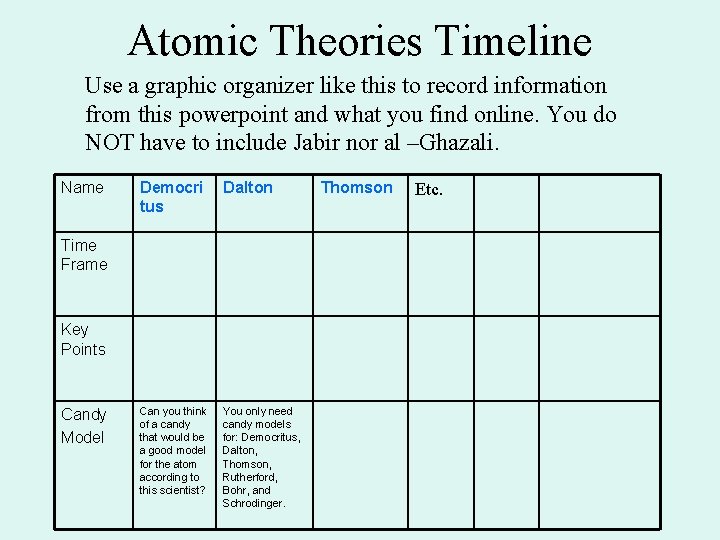
Atomic Theories Timeline Use a graphic organizer like this to record information from this powerpoint and what you find online. You do NOT have to include Jabir nor al –Ghazali. Name Democri tus Dalton Can you think of a candy that would be a good model for the atom according to this scientist? You only need candy models for: Democritus, Dalton, Thomson, Rutherford, Bohr, and Schrodinger. Time Frame Key Points Candy Model Thomson Etc.

Earliest alchemists- 2500 BC The Egyptians were engaged in practical chemistrymetallurgy, making dyes, and producing glass. There is no reference to an atom.

Kanada- India, 600 BC earliest recorded reference to the idea of an atom that I could find

Democritus 460 – 370 B. C. • There are various basic elements from which all matter is made • Everything is composed of small atoms moving in a void • Some atoms are round, pointy, oily, have hooks, etc. to account for their properties • Ideas rejected by leading philosophers because void = no existence

Simplified model- what doesn’t this show that makes his model distinct from Dalton’s? Video

Jabir- Arabic chemist of 8 th century • Considered one of the fathers of experimental chemistry but without atomisim

Al-Ghazali in the 11 th Century revived Islamic science

Alchemy reigned in western Europe

John Dalton 1766 -1844 • Introduced his ideas in 1803 • Each element is composed of extremely small particles called atoms • All the atoms of a given element are identical, but they differ from those of any other element • Atoms are neither created nor destroyed in any chemical reaction • A given compound always has the same relative numbers and kinds of atoms

Dalton’s Theory 1. Elements are made of tiny, indivisible particles (atoms).

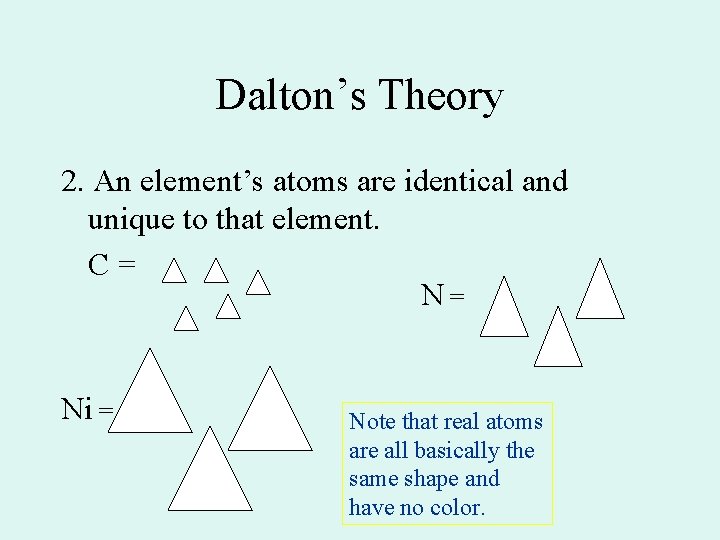
Dalton’s Theory 2. An element’s atoms are identical and unique to that element. C= N= Ni = Note that real atoms are all basically the same shape and have no color.

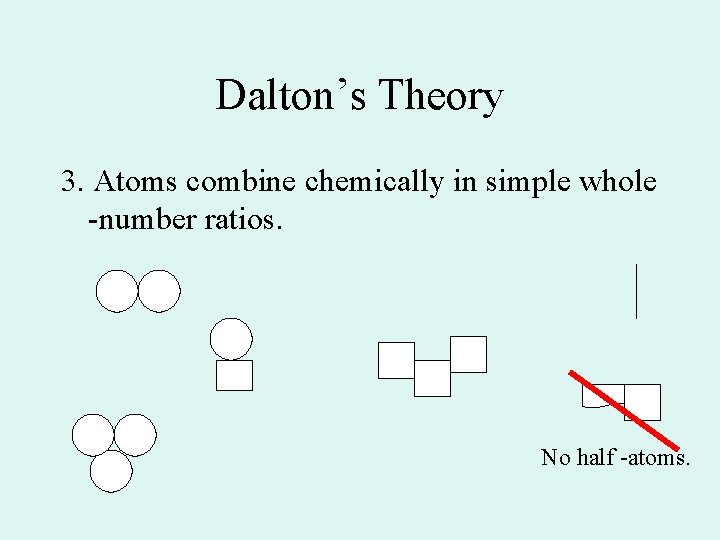
Dalton’s Theory 3. Atoms combine chemically in simple whole -number ratios. No half -atoms.

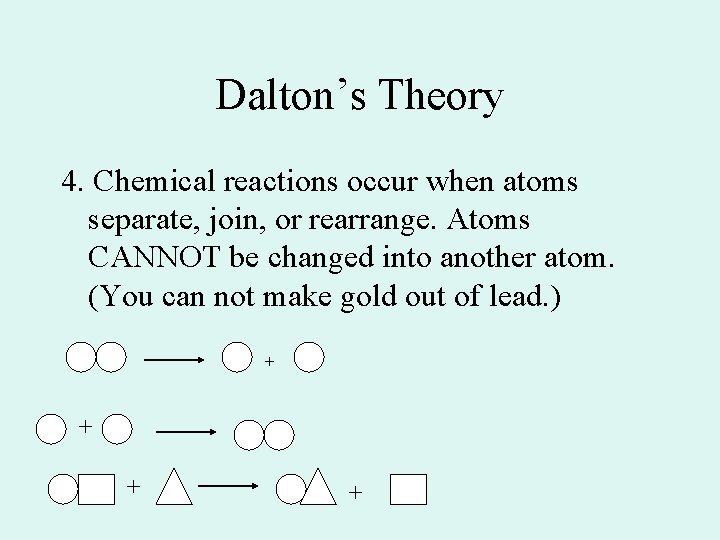
Dalton’s Theory 4. Chemical reactions occur when atoms separate, join, or rearrange. Atoms CANNOT be changed into another atom. (You can not make gold out of lead. ) + +

Now go back and see if you can make a distinction between Dalton’s and Democritus’ models.

J. J. Thomson 1856 -1940 • Discovered electron 1897 – Cathode Ray Experiment • Plum Pudding model 1904 – Electrons in a soup of positive charges • Discovered isotopes 1913

JJ Thomson’s Ideas

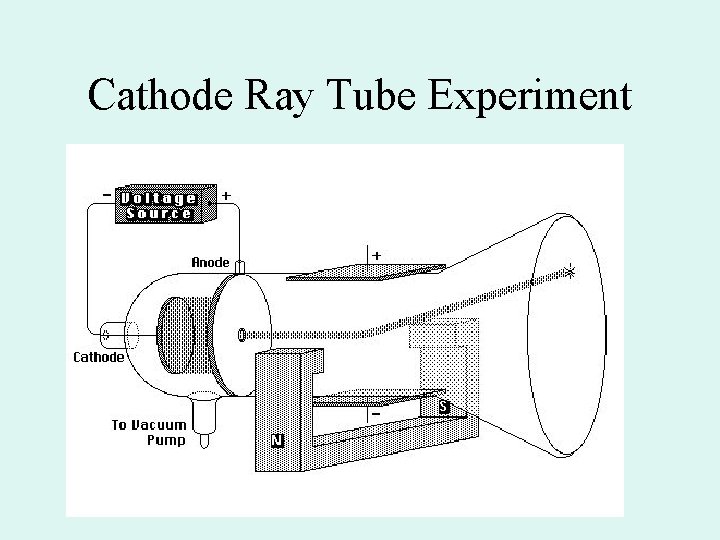
Experiments with Cathode Ray Tubes (late 1890 s)

Cathode Ray Tube Experiment

Deflection of the “ray” Evidence of the electron Video

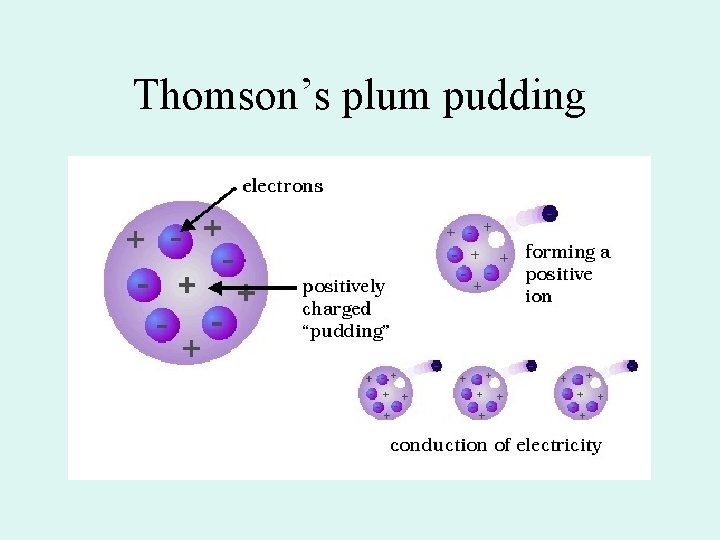
Thomson’s plum pudding

A real plum pudding

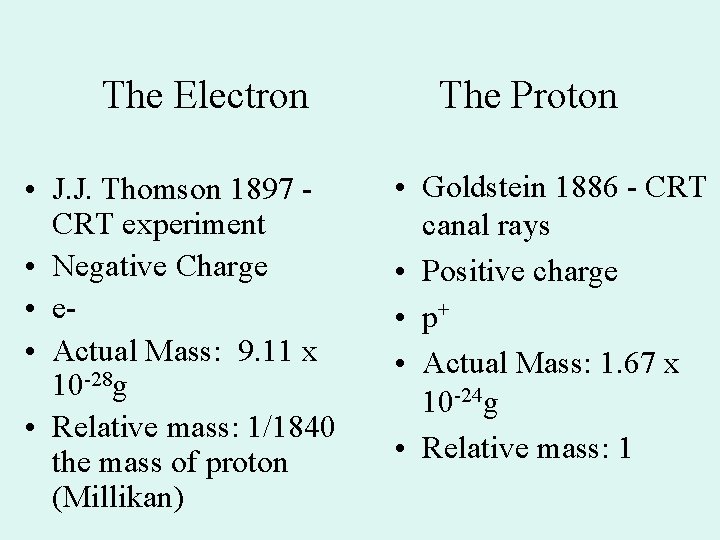
The Electron • J. J. Thomson 1897 CRT experiment • Negative Charge • Actual Mass: 9. 11 x 10 -28 g • Relative mass: 1/1840 the mass of proton (Millikan) The Proton • Goldstein 1886 - CRT canal rays • Positive charge • p+ • Actual Mass: 1. 67 x 10 -24 g • Relative mass: 1

Ernest Rutherford 1871 -1937 • Nucleus Theory 1910 – alpha particle gold foil experiment • An atom’s mass is mostly in the nucleus • The nucleus has a positive charge (Moseley) • Electrons in fixed orbit

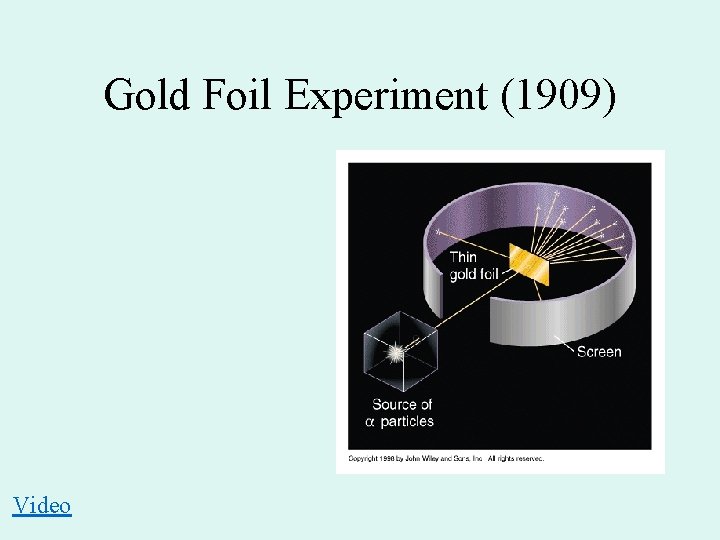
Gold Foil Experiment (1909) Video

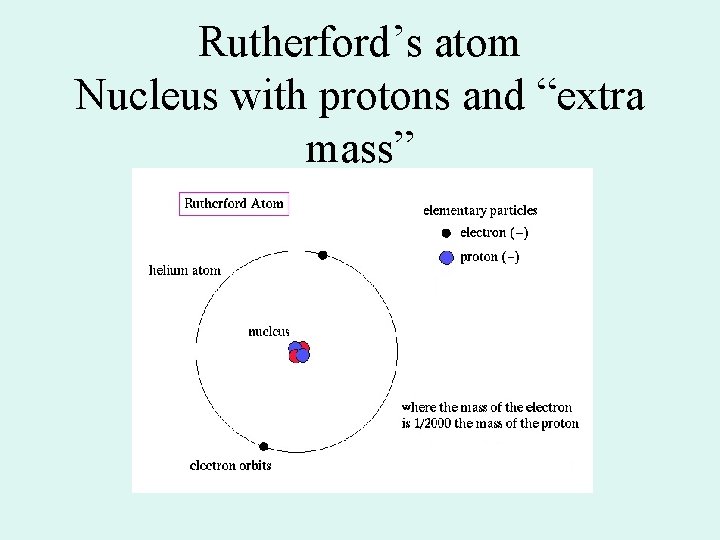
Rutherford’s atom Nucleus with protons and “extra mass”

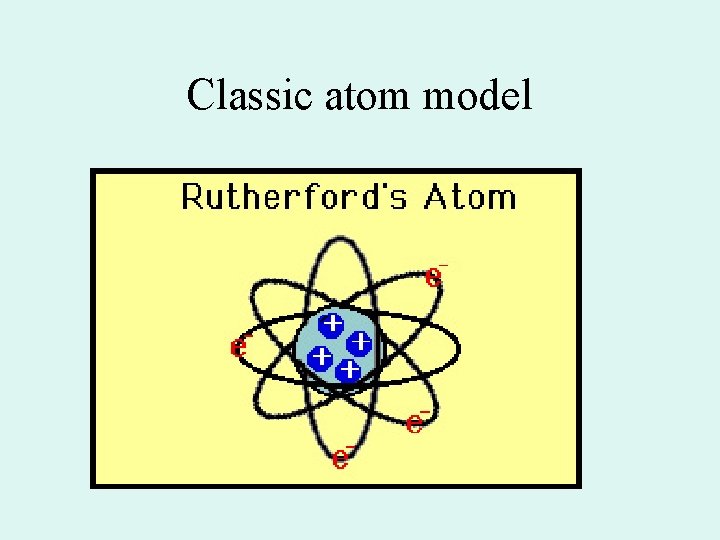
Classic atom model

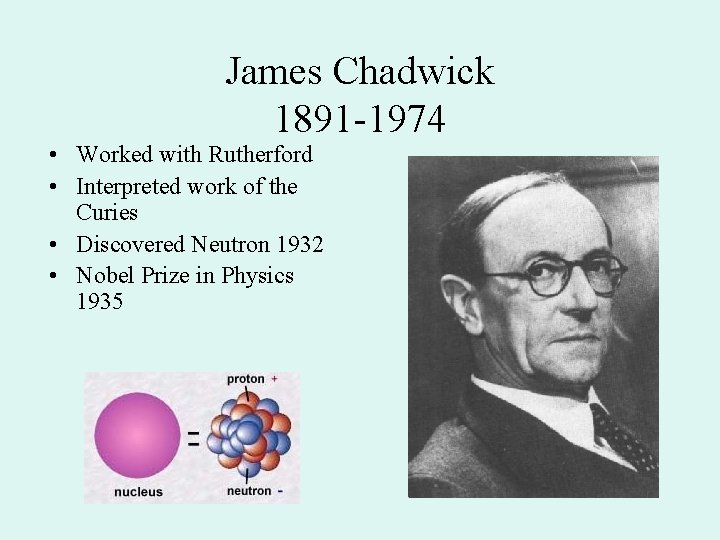
James Chadwick 1891 -1974 • Worked with Rutherford • Interpreted work of the Curies • Discovered Neutron 1932 • Nobel Prize in Physics 1935

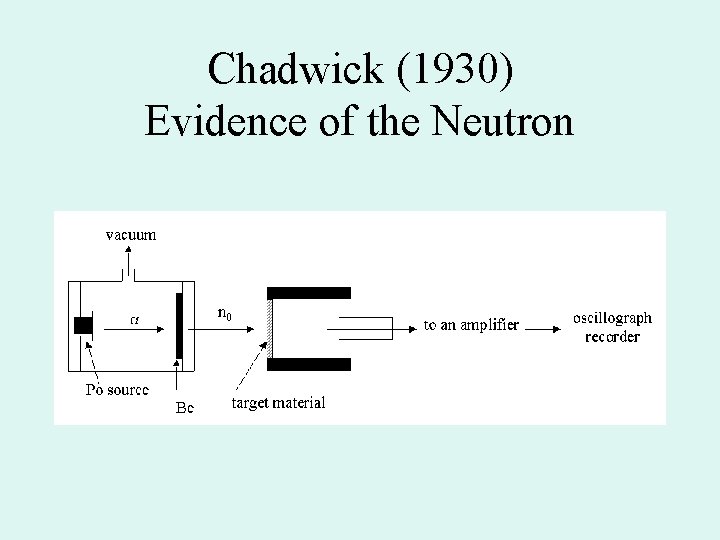
Chadwick (1930) Evidence of the Neutron

The Neutron • • • Chadwick 1932 - nuclear bombardment No charge n 0 Actual Mass: 1. 67 x 10 -24 g Relative Mass: 1

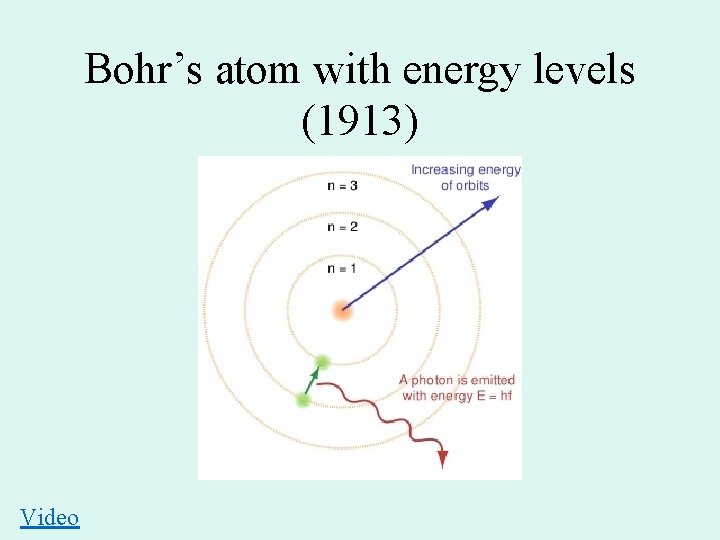
Niels Bohr 1885 -1962 • Planetary Model 1913 – Nucleus surrounded by orbiting electrons at different energy levels – Electrons have definite orbits • Utilized Planck’s Quantum Energy theory • Worked on the Manhattan Project (US atomic bomb)

Bohr’s atom with energy levels (1913) Video

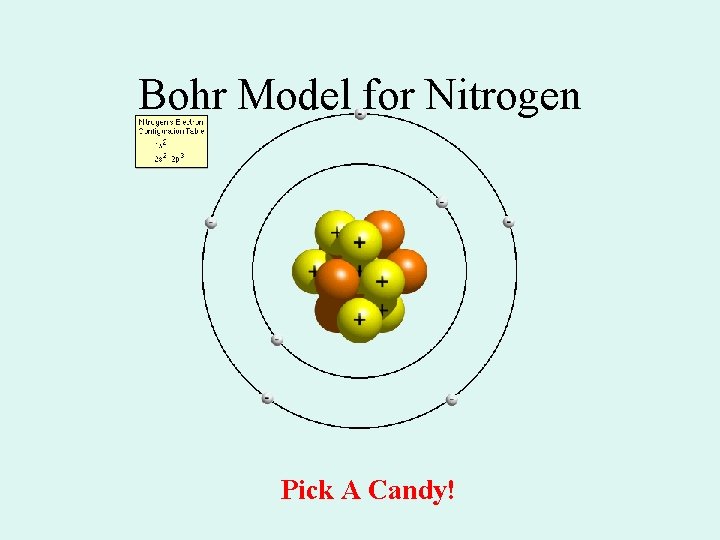
Bohr Model for Nitrogen Pick A Candy!

Quantum mechanical model • Bohr’s model was good for hydrogen, but failed with atoms with more electrons • Schrodinger used new experimental results to come up with a mathematical equation that explains electron behavior • NO exact paths to follow- more of a cloud where electrons may be found • Video

Ernst Schrödinger 1887 -1961 Werner Heisenberg 1901 -1976 • Quantum Mechanical Model 1926 – Electrons are in probability zones called “orbitals”, not orbits and the location cannot be pinpointed – Electrons are particles and waves at the same time – Developed quantum numbers based on theories of Einstein and Planck

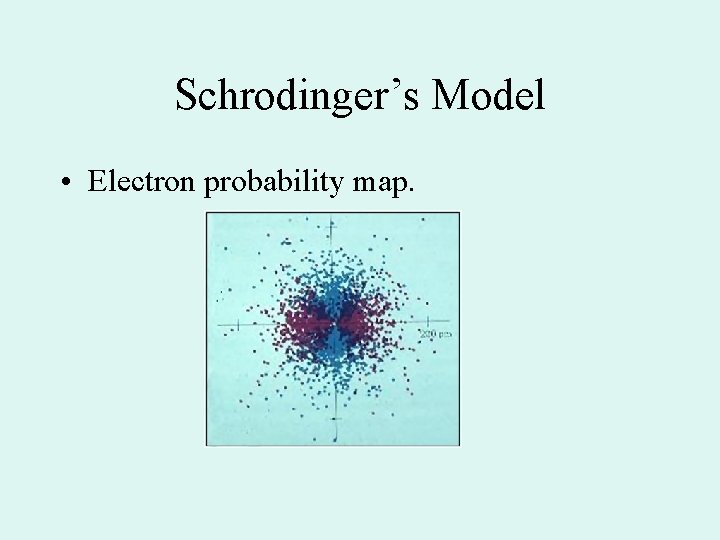
Schrodinger’s Model • Electron probability map.

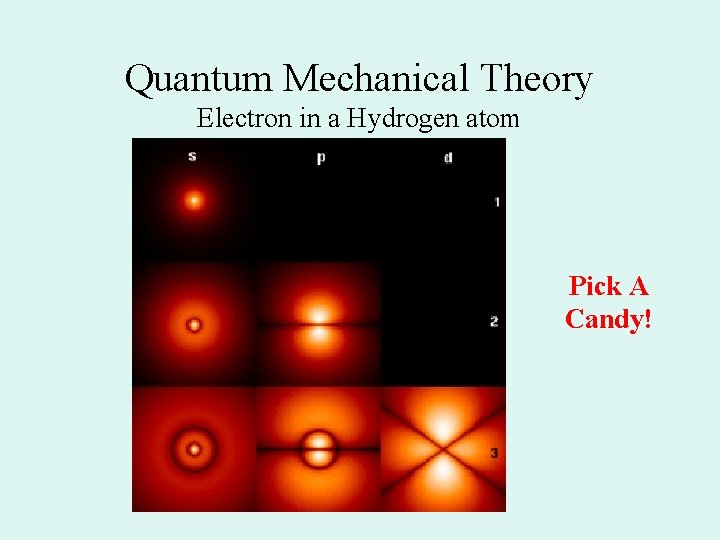
Quantum Mechanical Theory Electron in a Hydrogen atom Pick A Candy!

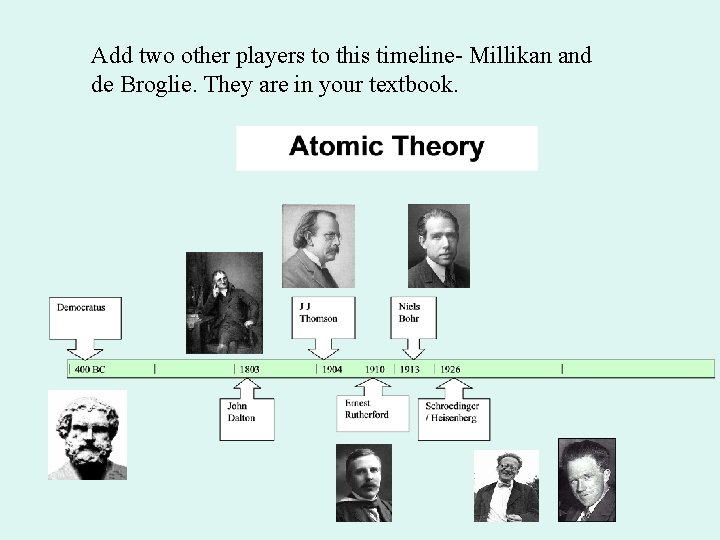
Add two other players to this timeline- Millikan and de Broglie. They are in your textbook.

Energy levels • Electrons can only be at certain energy levels • Quantum- amount of energy needed to move from one energy level to the next • Cannot stop between levels

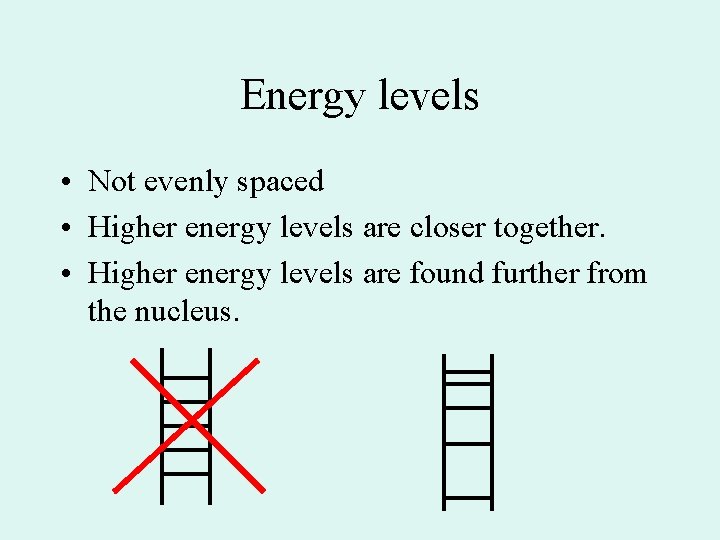
Energy levels • Not evenly spaced • Higher energy levels are closer together. • Higher energy levels are found further from the nucleus.

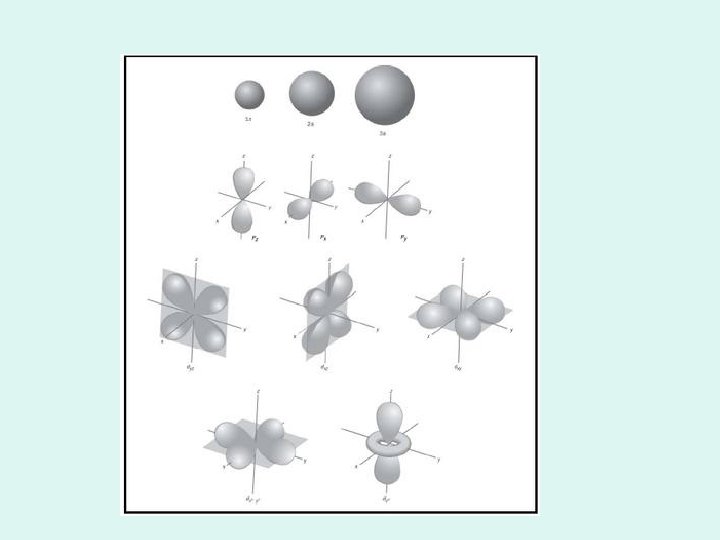
Atomic orbitals • A region of space where there is a high probability of finding an electron • Defined mathematically by Schrodinger • Each orbital has a different shape

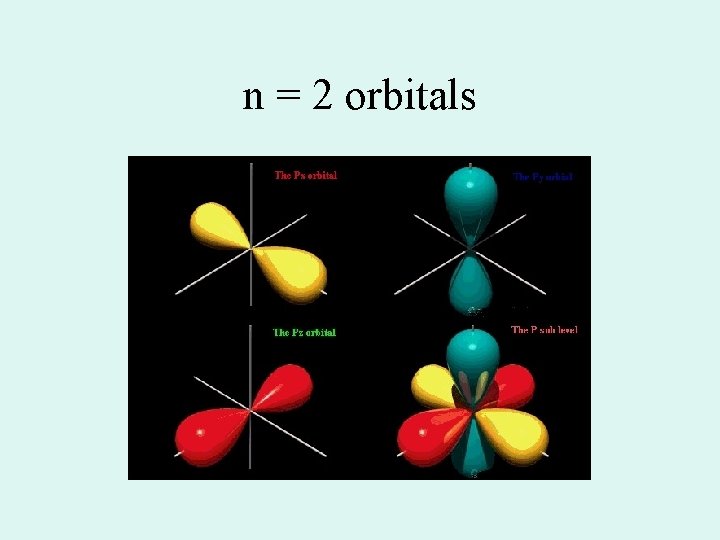
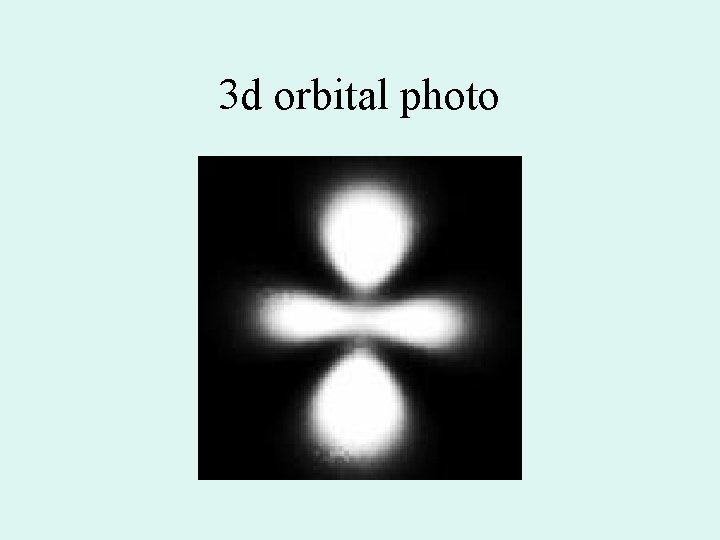
Orbital shapes • s = spherical • p= dumb bell on three axes • d= like a double p on three axes and an odd (one dumbbell with a donut) • S, p, and d are also the names of the sublevels

n = 2 orbitals


3 d orbital photo