ATOMIC STRUCTURE Atomic History What is an Atom














- Slides: 25

ATOMIC STRUCTURE: Atomic History

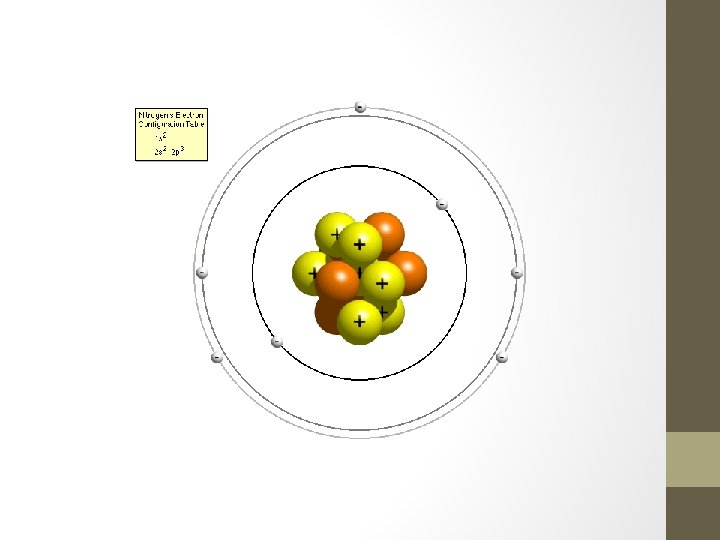
What is an Atom • Smallest part of matter that exists • Retains the properties of the element that it is a part of • Cannot be broken down any further • Consists of at least one electron, proton, and neutron


Dalton’s Atomic Theory of Matter

Dalton’s Atomic Theory Has 4 Points • 1. All elements are composed of matter • 2. All atoms of an element are identical • 3. Atoms of different elements are made up of different materials • 4. Compounds are formed by combining atoms of different elements

People Associated With Discovering Atomic Structure

JJ Thomson “Plumb-Pudding Model” • Around 1897 • Credited with discovering the electron • Famous experiment is the “Cathode-Ray Tube Experiment” • Theory was that all of the charge of an atom is in a dense area located in the middle of the structure



Rutherford “Gold-Foil Experiment” • Around 1910 ish • Credited with discovering the nucleus of an atom • Used the Gold Foil Experiment to determine the location and charge of an atom’s nucleus

What is the Gold-Foil Experiment? • Major experiment in chemistry • Shot alpha-particles at gold foil and observed what happened • Basically 2 main things were observed: • 1. Most of the alpha particles went through the gold foil and hit the phosphor screen behind the foil • 2. Some of the alpha particles hit some unseen object and bounced back or in random directions away from the gold foil



What are the conclusions of the Experiment? • 1. Atoms are mostly empty space: Have large volume • 2. Atoms have a dense positive core called the nucleus and this is where the mass of the atom is located.

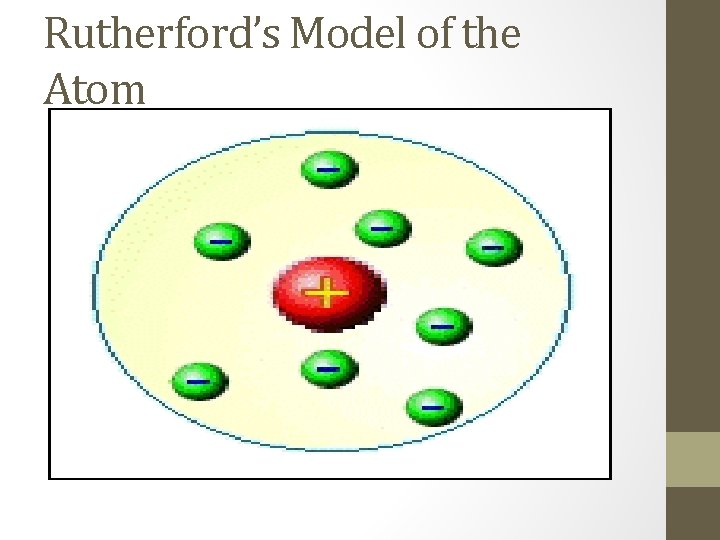
Rutherford’s Model of the Atom


What is wrong with the model? • Electrons are protons are together in the middle of the atom • Can’t happen due to the attractions that take place between the oppositely charged particles • Had to be a better model that somehow separated the two particles from each other.

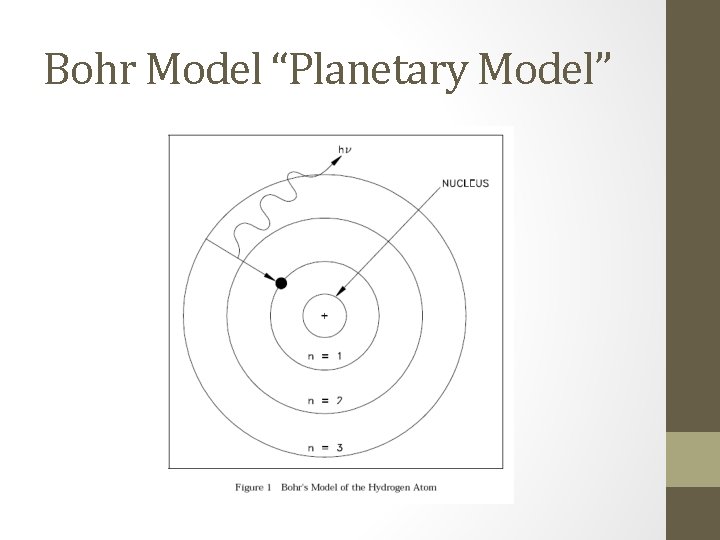
Bohr Model “Planetary Model”

What is the model • Uses the Rutherford model as its basis • Around 1915 ish • Places the electrons outside of the nucleus away from the protons • Electrons are in orbits around the nucleus similar to the way the planets rotate around the sun • All of model is OK EXCEPT for 1 key fact


What is correct/incorrect about the model? • Correct: 1. Nucleus 2. Electrons in orbits away from nucleus 3. Protons in nucleus Incorrect: 1. Electrons are not stationary in orbits 2. Electrons can move around within an orbit to any random spot that they find, and can also move between levels within an atom


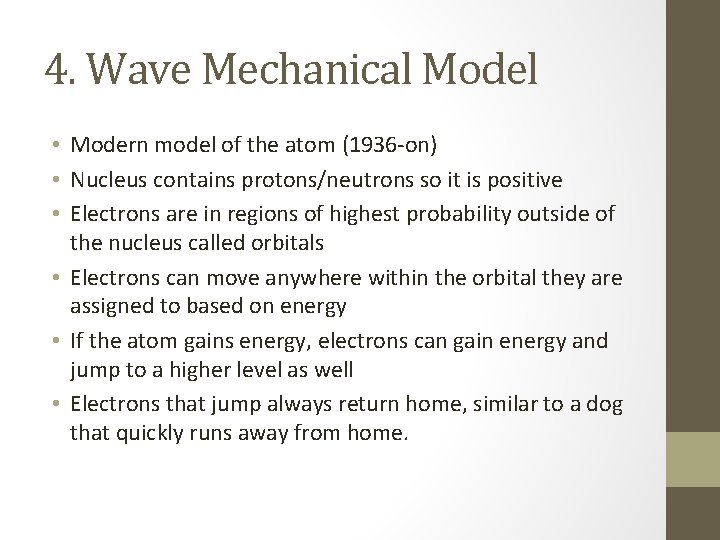
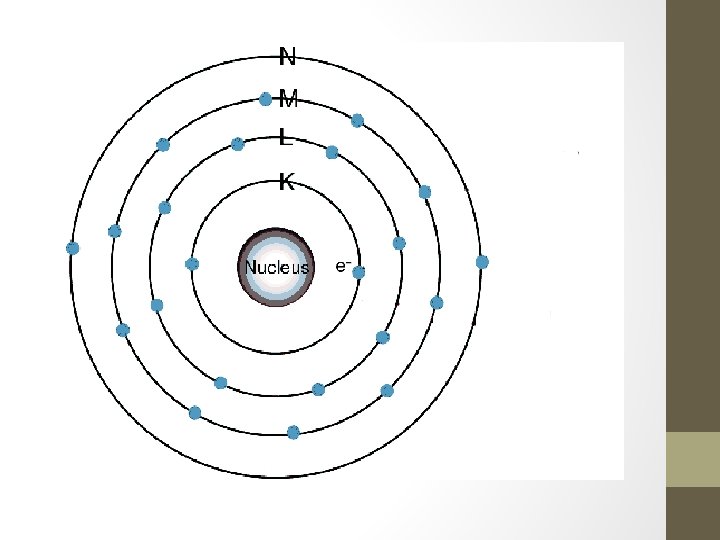
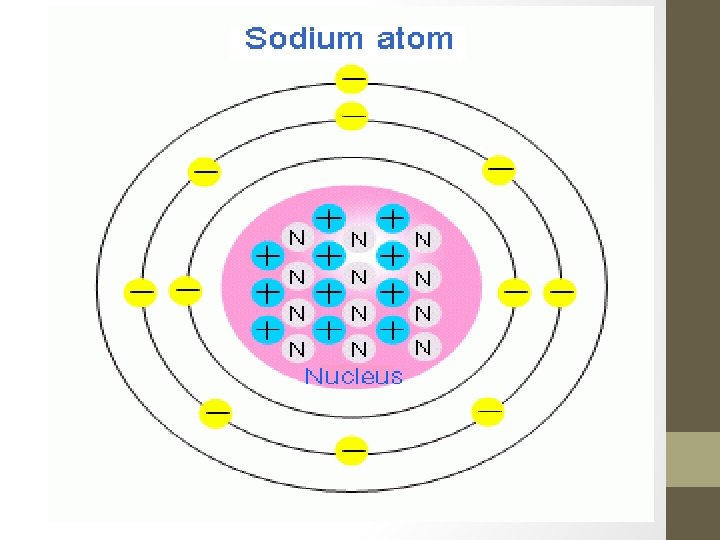
4. Wave Mechanical Model • Modern model of the atom (1936 -on) • Nucleus contains protons/neutrons so it is positive • Electrons are in regions of highest probability outside of the nucleus called orbitals • Electrons can move anywhere within the orbital they are assigned to based on energy • If the atom gains energy, electrons can gain energy and jump to a higher level as well • Electrons that jump always return home, similar to a dog that quickly runs away from home.

