Atomic Theory Atomic History n n n n









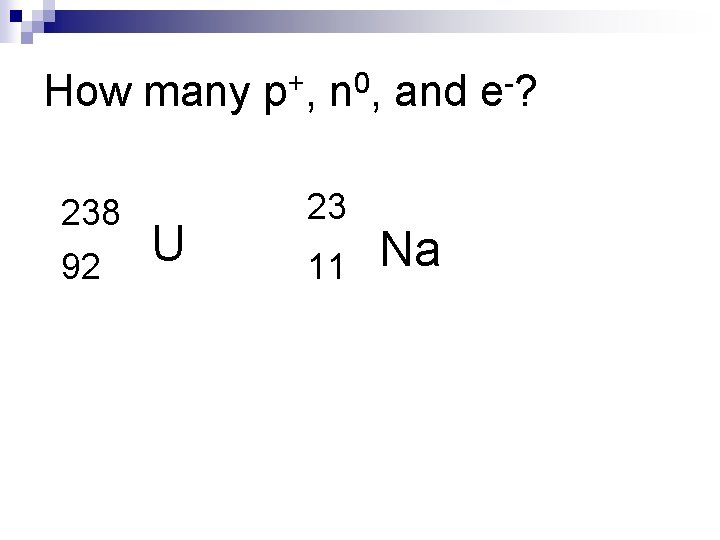


- Slides: 22

Atomic Theory

Atomic History n n n n Ancient Greeks John Dalton JJ Thomson Ernest Rutherford James Chadwick Neils Bohr Erwin Schrödinger

Ancient Greeks (~ 400 B. C. E) n Democritus (460 – 370 B. C. E. ) ¨ n All matter is made of tiny, indestructible units called ATOMOS Aristotle (384 – 322 B. C. E) & Plato (428 – 348 B. C. E) ¨ Completely disagreed with Democritus. Public opinion sided with these guys that all matter was made of EARTH, AIR, FIRE, & WATER

John Dalton (1766 – 1844) & Dalton’s Atomic Theory n John Dalton Chemist & Physicist who made a living teaching ¨ His theory is backed by many experiments ¨ n Theory ¨ ¨ ¨ Elements are made of particles called atoms All atoms of an element are identical Atoms of 1 element are diff. from atoms of another element Atoms of elements can combine to form compounds with simple, whole number ratios Atoms can’t be destroyed or created, they’re just rearranged in a chemical reaction

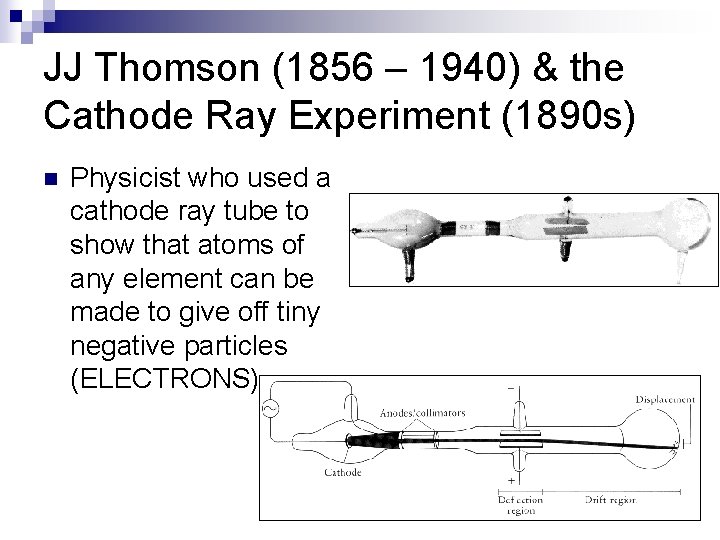
JJ Thomson (1856 – 1940) & the Cathode Ray Experiment (1890 s) n Physicist who used a cathode ray tube to show that atoms of any element can be made to give off tiny negative particles (ELECTRONS)

JJ’s Model- The Plum Pudding Model (Chocolate Chip Cookie anyone? )

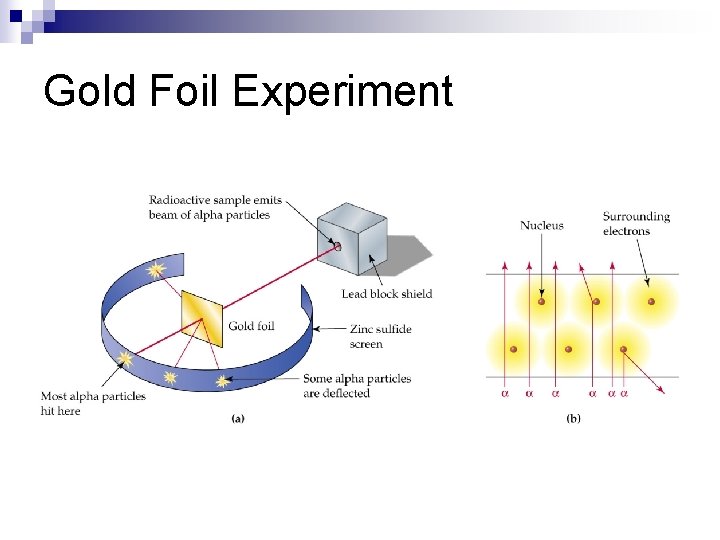
Ernest Rutherford (1871 – 1937) & Gold Foil Experiment (1911) n Ernest ¨ Expected the positive α (alpha) particles to pass straight through to the back of the detector. He was surprised when they bounced off at strange angles. ¨ He surmised that the alpha particles were bouncing off a small + charge in the Gold atoms. He called that part the NUCLEUS!

Gold Foil Experiment

Some More Stuff Ernie Did n In 1918 – He experimented by bombarding N 2(g) with alpha particles. 1 of the results was that a whole lot of H 2(g) was created. What’s going on? ¨ Ernie figured out that the H atoms must have come from inside the N 2. That means that ATOMS ARE DIVISIBLE!!!! He eventually isolated those H atoms and discovered they were actually PROTONS

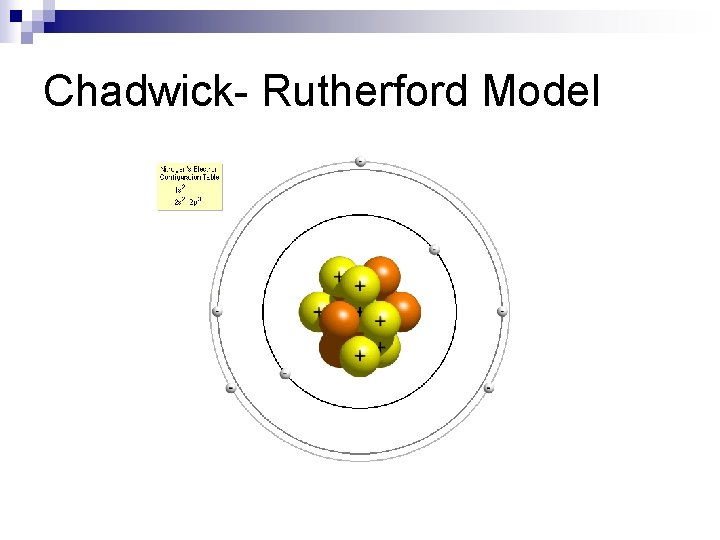
Ernie found a friend- James Chadwick (1932). n Ernie’s assistant, James Chadwick found the NEUTRON in 1932. ¨ He put some Be in a chamber with Po (gives off alpha particles). The alpha particles hit the Be which gave off some particles at high, high speeds. At first he thought they were gamma (γ) rays but they moved too fast for gamma rays. He worked his way thru some calculations and discovered they were not, but some neutral particles that he named (NEUTRONS)

Chadwick- Rutherford Model

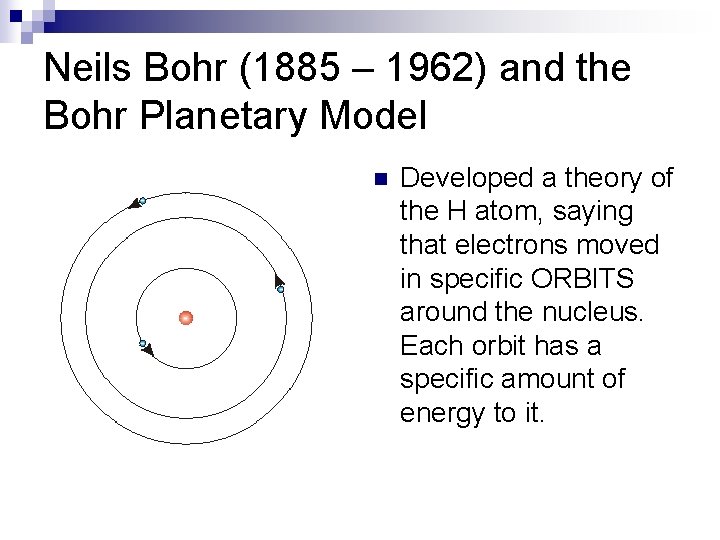
Neils Bohr (1885 – 1962) and the Bohr Planetary Model n Developed a theory of the H atom, saying that electrons moved in specific ORBITS around the nucleus. Each orbit has a specific amount of energy to it.

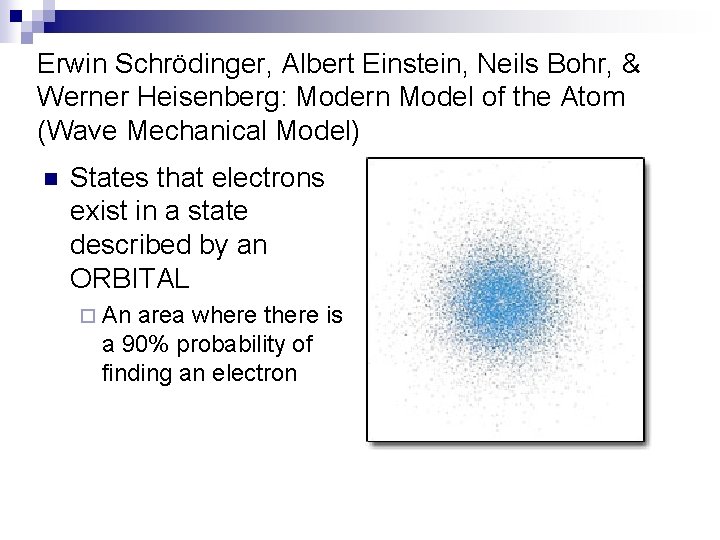
Erwin Schrödinger, Albert Einstein, Neils Bohr, & Werner Heisenberg: Modern Model of the Atom (Wave Mechanical Model) n States that electrons exist in a state described by an ORBITAL ¨ An area where there is a 90% probability of finding an electron

ATOMIC STRUCTURE Physical structure n Atomic Number n Mass Number n Isotopes n Atomic Mass n Calculating Atomic Mass n

Atomic Structure n n An atom is the defining part of what makes an element, an element. Cannot be broken down chemically. Atoms are mostly empty space! Atoms are very, very small! If an atom were 2 miles wide, the nucleus would be the size of a baseball!

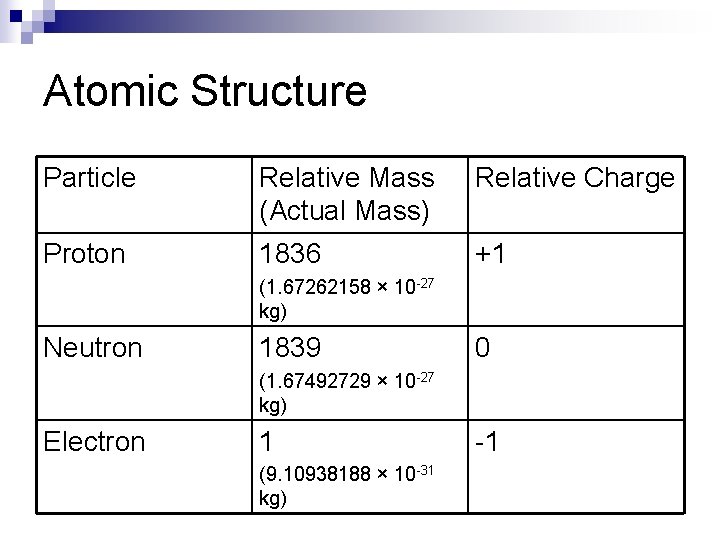
Atomic Structure Particle Relative Mass (Actual Mass) Relative Charge Proton 1836 +1 (1. 67262158 × 10 -27 kg) Neutron 1839 0 (1. 67492729 × 10 -27 kg) Electron 1 (9. 10938188 × 10 -31 kg) -1

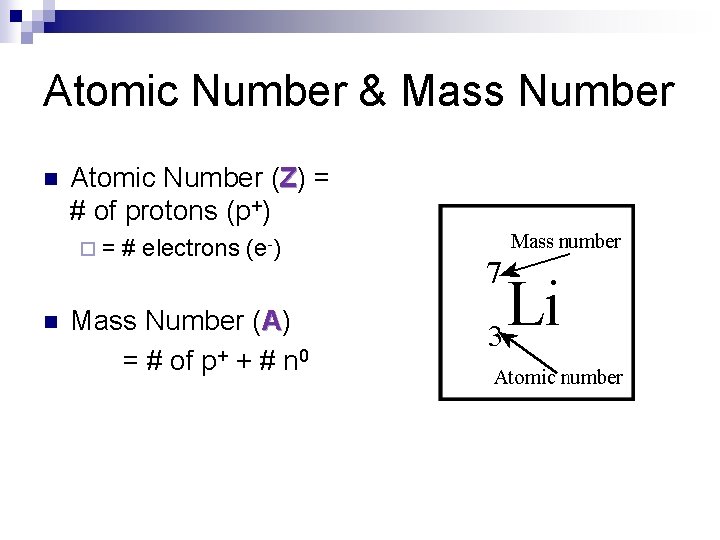
Atomic Number & Mass Number n Atomic Number (Z) = # of protons (p+) ¨= n # electrons (e-) Mass Number (A) = # of p+ + # n 0
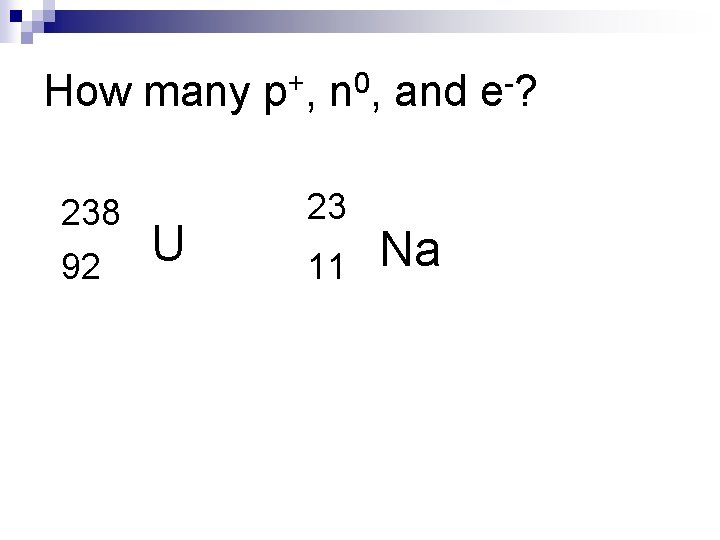
How many p+, n 0, and e-? 238 92 U 23 11 Na

Isotopes n n Atoms of an element that have different #’s of neutrons. An element is composed of varying percentages (abundances) of isotopes

Isotopes n Examples ¨ 1 1 H 2 1 H ¨ 6 3 Li 7 3 Li 3 1 H

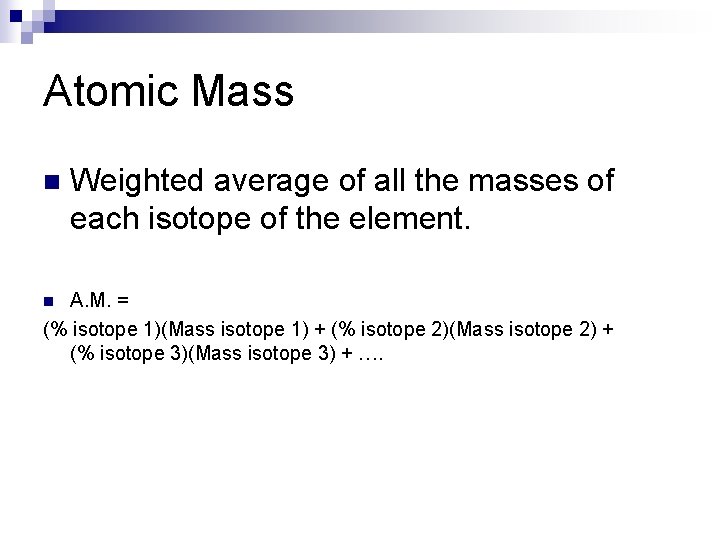
Atomic Mass n Weighted average of all the masses of each isotope of the element. A. M. = (% isotope 1)(Mass isotope 1) + (% isotope 2)(Mass isotope 2) + (% isotope 3)(Mass isotope 3) + …. n

Examples n Calculate the average atomic mass of gold ¨ 50% of 197 Au weighs 197 ¨ 50% of 198 Au weighs 198