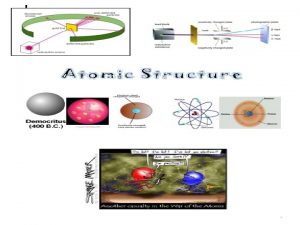
The Development of the Atomic Theory Democritus to





















- Slides: 34

The Development of the Atomic Theory Democritus to Rutherford

THE PERIODIC TABLE ü Use it all year. ü Has ions on the backside for later.

What do you know about the atom?

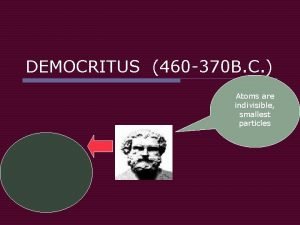
450 BC Greek - Democritus Atom was indivisible Theorized the existence of the atom Also, theorized that there were just four 'elements' - fire, water, air, earth

1760 Benjamin Franklin discovers that an object can have an electrical charge. Charge can be positive or negative

Laserdisc: Electroscope

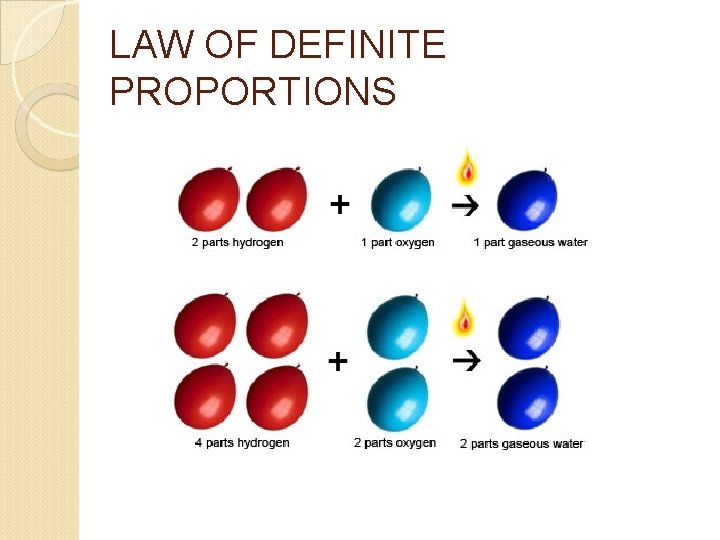
1803 John Dalton Atom was indivisible All elements are composed of atoms The same atoms for one element are exactly alike Atoms are neither created or destroyed in a chemical reaction In a chemical reaction, atoms are separated, combined, or rearranged Different atoms combine in simple whole number ratios to form compounds (Law of Definite Proportions)

LAW OF DEFINITE PROPORTIONS

THE DIVISIBLE ATOM Up until this point, the atom: Is indivisible Composes all elements The same atoms for one element are exactly alike Atoms are neither created or destroyed in a chemical reaction Atoms are separated, combined, or rearranged in a chemical reaction in simple whole number ratios.

1880 Sir William Crookes determined that rays were traveling from one end to another in the cathode ray tube

CATHODE RAY TUBE others experimented with the cathode ray tube and discovered that the type of gas had no effect so the ray must be a part of all matter. A magnet deflected the ray, so it must be composed of charged particles, and it deflected toward the positive, so the charged particles must be negative.

Late 1890 s J. J. Thomson discovered the electron using the cathode ray tube determined that the electron was smaller than a hydrogen atom. Knew the atom was neutral and the electron was negative, so there must be positive material with a lot more mass.

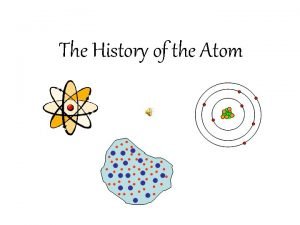
J. J. THOMSON Said the atom was a positive puddinglike material throughout which negatively charged electrons were scattered - Plum Pudding or Chocolate Chip Cookie Model

1909 Robert Millikan Knew the electron was negative, but actually determined the charge (as a measurement). Was able to use this measurement to determine the mass of an electron. It was 1/1840 of a hydrogen atom

LASERDISC:

LASERDISC 1. What is an alpha particle? 1. What did Rutherford expect to see? What did he actually see? 1. What is Rutherford’s model of the atom?

1909 Ernest Rutherford Did a famous gold foil experiment (the alpha scattering experiment) Calculated that the atom was mostly empty space through which electrons move. Concluded that the atom has a small, dense, positively charged, centrallylocated nucleus surrounded by negatively charged electrons

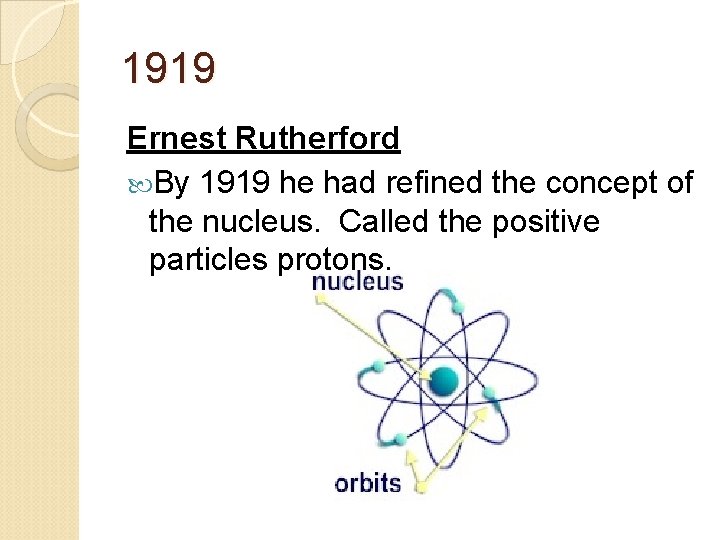
1919 Ernest Rutherford By 1919 he had refined the concept of the nucleus. Called the positive particles protons.

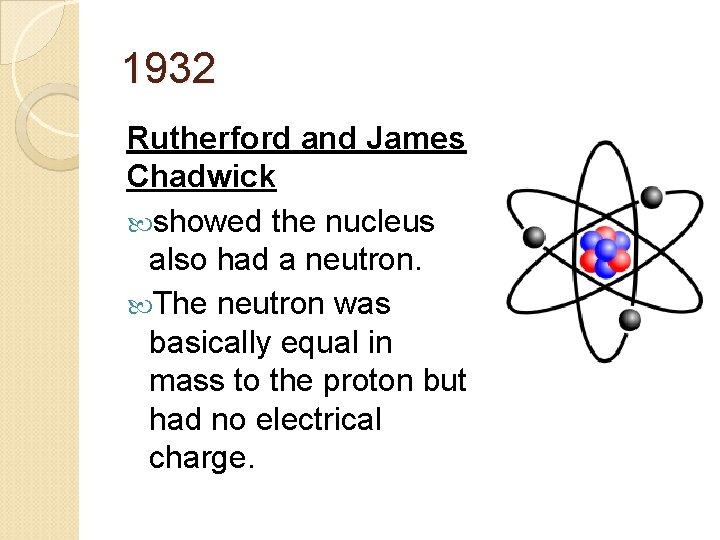
1932 Rutherford and James Chadwick showed the nucleus also had a neutron. The neutron was basically equal in mass to the proton but had no electrical charge.

THE DIVISIBLE ATOM Is indivisible FALSE Composes all elements TRUE The same atoms for one element are exactly alike FALSE Atoms are neither created or destroyed in a chemical reaction TRUE Atoms are separated, combined, or rearranged in a chemical reaction in simple whole number ratios. TRUE

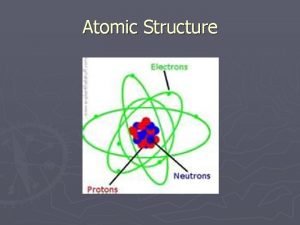
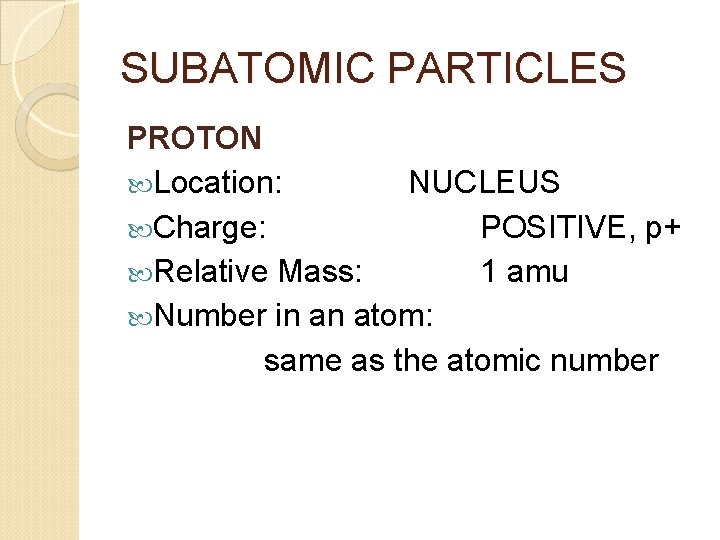
SUBATOMIC PARTICLES PROTON Location: NUCLEUS Charge: POSITIVE, p+ Relative Mass: 1 amu Number in an atom: same as the atomic number

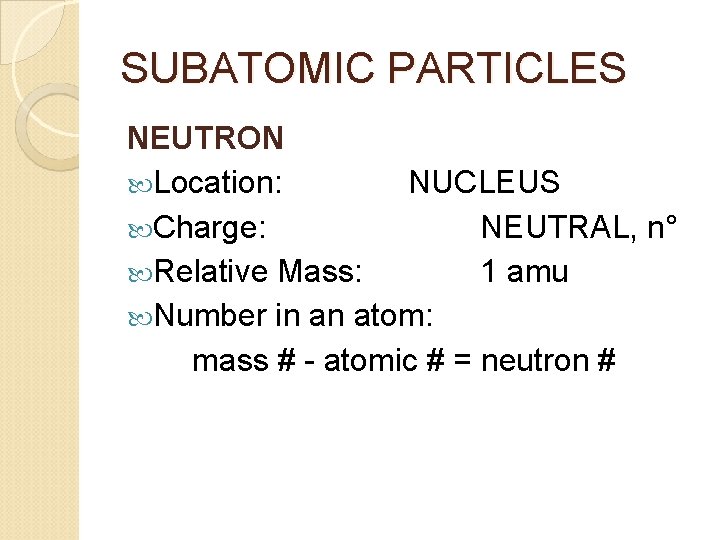
SUBATOMIC PARTICLES NEUTRON Location: NUCLEUS Charge: NEUTRAL, n° Relative Mass: 1 amu Number in an atom: mass # - atomic # = neutron #

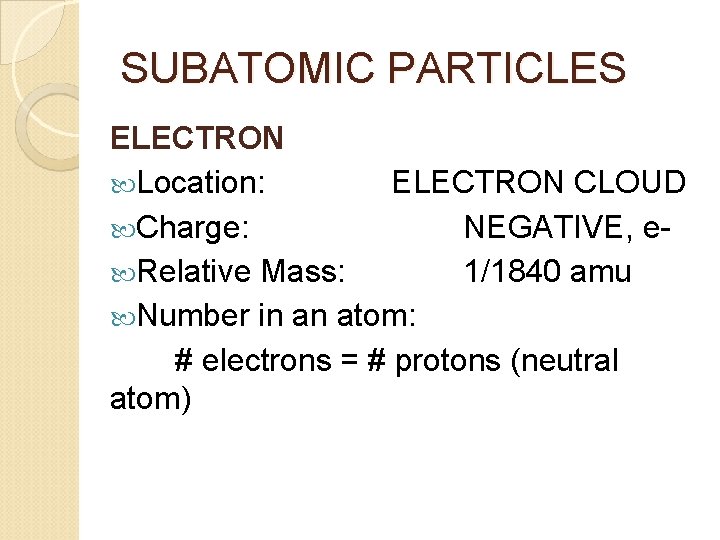
SUBATOMIC PARTICLES ELECTRON Location: ELECTRON CLOUD Charge: NEGATIVE, e Relative Mass: 1/1840 amu Number in an atom: # electrons = # protons (neutral atom)

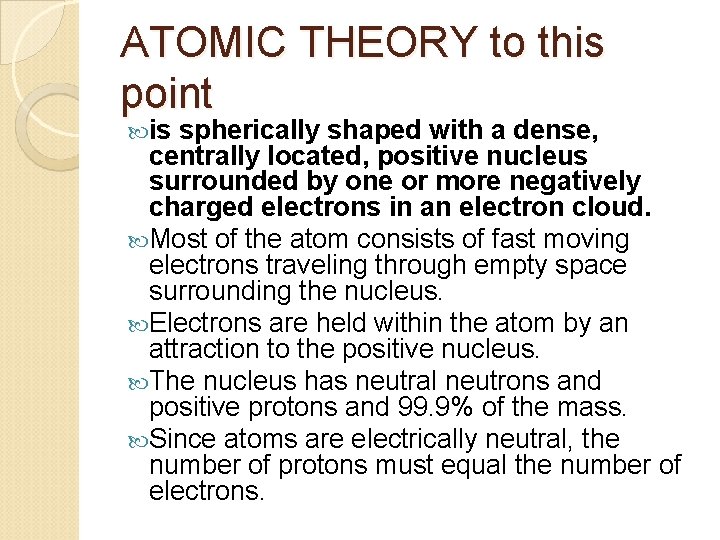
ATOMIC THEORY to this point is spherically shaped with a dense, centrally located, positive nucleus surrounded by one or more negatively charged electrons in an electron cloud. Most of the atom consists of fast moving electrons traveling through empty space surrounding the nucleus. Electrons are held within the atom by an attraction to the positive nucleus. The nucleus has neutral neutrons and positive protons and 99. 9% of the mass. Since atoms are electrically neutral, the number of protons must equal the number of electrons.

1912 Henry Moseley Discovered that atoms of each element contain a unique positive charge. The number of protons in an atom identifies it.

IMPORTANT NUMBERS ATOMIC NUMBER = number of protons (and number of electrons). It is unique for each element MASS NUMBER = the number of neutrons plus the number of protons (nucleons). It is the whole number of the atomic mass. Changes with isotopes.

PRACTICE How many protons and electrons are present in a vanadium atom? 2. How many protons and electrons are present in a nitrogen atom? 3. How many protons and electrons are present in an argon atom? 4. What is the name of the element that has atoms that contain 5 protons? 1.

PRACTICE How many protons, neutrons, and electrons are present in zirconium 91? 6. How many protons, neutrons, and electrons are present in cesium-140? 7. What is the atomic number and the mass number for the element with 27 protons and 32 neutrons? 8. What is the atomic number and mass number for the element with 84 protons and 125 neutrons? 5.

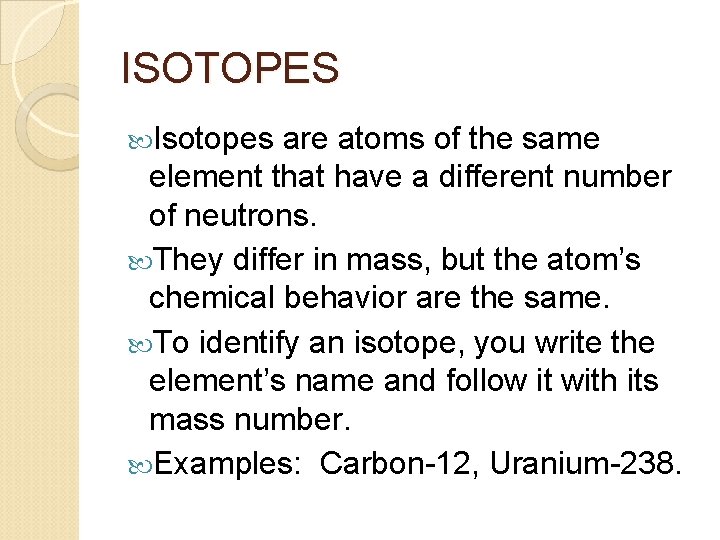
ISOTOPES Isotopes are atoms of the same element that have a different number of neutrons. They differ in mass, but the atom’s chemical behavior are the same. To identify an isotope, you write the element’s name and follow it with its mass number. Examples: Carbon-12, Uranium-238.

PRACTICE 1. Element D Element F 2. Element J Element L 3. Element X Element Y 4. Element Q Element R 5. Element T Element Z 6. Element W Element V 7. Element P Element S 6 p+ and 7 n 7 p+ and 7 n 27 p+ and 32 n 27 p+ and 33 n 17 p+ and 18 n 18 p+ and 17 n 56 p+ and 81 n 56 p+ and 82 n Z = 20 and A = 40 Z = 20 and A = 41 8 p+ and 8 n 7 p+ and 8 n Z = 92 and A = 238 92 p+ and 143 n

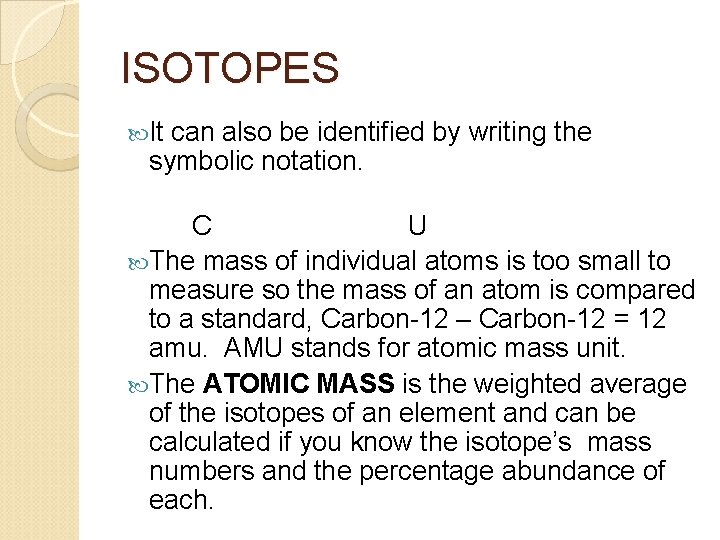
ISOTOPES It can also be identified by writing the symbolic notation. C U The mass of individual atoms is too small to measure so the mass of an atom is compared to a standard, Carbon-12 – Carbon-12 = 12 amu. AMU stands for atomic mass unit. The ATOMIC MASS is the weighted average of the isotopes of an element and can be calculated if you know the isotope’s mass numbers and the percentage abundance of each.

PRACTICE The atomic mass of iridium-191 is 191. 0 amu and iridium-193 is 193. 0 amu. The percentage abundance for each is 37. 58% (iridium-191) and 62. 62% (iridium-193). Calculate the average atomic mass of iridium. Show your work.

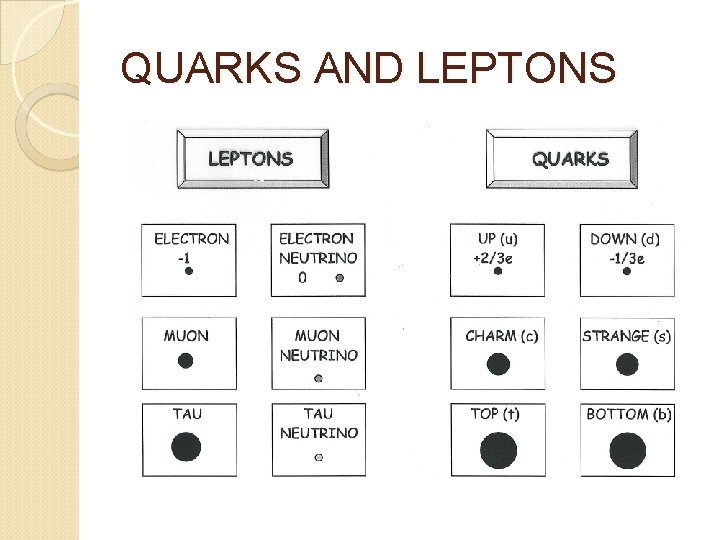
QUARKS AND LEPTONS

PROTONS AND NEUTRONS PROTON NEUTRON
 Atomic model diagram democritus
Atomic model diagram democritus Democritus
Democritus Timeline of democritus
Timeline of democritus Democritus atomic theory model
Democritus atomic theory model Atomic model timeline
Atomic model timeline Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach Democritus atomic model
Democritus atomic model Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Periodic trends
Periodic trends Radius size trend
Radius size trend Isotope abundance formula
Isotope abundance formula Atomic
Atomic Atomic number vs atomic radius
Atomic number vs atomic radius Demokritos atom modeli
Demokritos atom modeli Democritus atom model
Democritus atom model Democritus contribution
Democritus contribution Aristotle democritus
Aristotle democritus Democritus dalton
Democritus dalton What scientist discovered the electron
What scientist discovered the electron Democritus milky way
Democritus milky way Democritus and dalton
Democritus and dalton Democritus
Democritus Democritus atom modeli
Democritus atom modeli Nick the camel mnemonic
Nick the camel mnemonic Teori dalton
Teori dalton Atom table of contents
Atom table of contents Gold foil experiment
Gold foil experimentSir james chadwick atomic theory
 Democritus 400 bc
Democritus 400 bc Democritus dalton thomson rutherford and bohr
Democritus dalton thomson rutherford and bohr What did democritus propose
What did democritus propose Teori atom democritus
Teori atom democritus Democritus
Democritus Greek philosopher democritus
Greek philosopher democritus Democritus 460 bc
Democritus 460 bc